247537
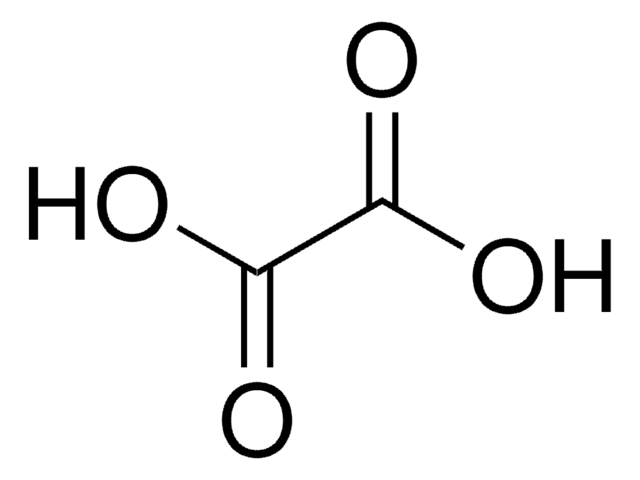
Oxalic acid dihydrate
ACS reagent, ≥99%
Synonym(s):
Ethanedioic acid dihydrate
About This Item
Recommended Products
grade
ACS reagent
Quality Level
vapor density
4.4 (vs air)
vapor pressure
<0.01 mmHg ( 20 °C)
assay
≥99%
99.5-102.5% (ACS specification)
form
solid
impurities
H2SO4, passes test (darkened)
≤0.001% N compounds
≤0.005% insolubles
ign. residue
≤0.01%
mp
104-106 °C (lit.)
anion traces
chloride (Cl-): ≤0.002%
sulfate (SO42-): ≤0.005%
cation traces
Ca: ≤0.001%
Fe: ≤2 ppm
heavy metals: ≤5 ppm (by ICP-OES)
functional group
carboxylic acid
SMILES string
[H]O[H].[H]O[H].OC(=O)C(O)=O
InChI
1S/C2H2O4.2H2O/c3-1(4)2(5)6;;/h(H,3,4)(H,5,6);2*1H2
InChI key
GEVPUGOOGXGPIO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a catalyst in the preparation of tetrahydroquinoline derivatives via imino Diels-Alder reaction.
- As a homogeneous catalyst in the preparation of highly functionalized piperidines via multi-component reaction.
- As an oxidant for the formation of triazolinediones from corresponding urazoles and bisurazoles via oxidation.
- In the transformation of thiols to nitrosothiols by reacting with sodium nitrite.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service