AB5940
Anti-BACE Antibody, CT
Chemicon®, from rabbit
Synonym(s):
ASP2, BACE1, Beta Secretase, beta-Site APP Cleaving Enzyme
About This Item
Recommended Products
biological source
rabbit
Quality Level
antibody form
affinity isolated antibody
antibody product type
primary antibodies
clone
polyclonal
purified by
affinity chromatography
species reactivity
human
manufacturer/tradename
Chemicon®
technique(s)
immunohistochemistry: suitable
western blot: suitable
NCBI accession no.
UniProt accession no.
shipped in
wet ice
target post-translational modification
unmodified
Gene Information
human ... BACE1(23621)
General description
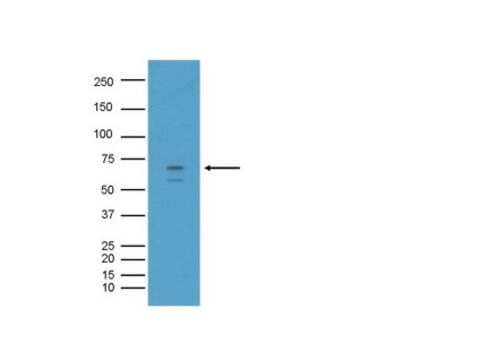
BACE is an N-glycosylated integral membrane aspartyl protease with Mr=70 kDa. Mature BACE is produced from the immature form through a series of post-translational proteolytic cleavages and glycosylation. Sequence analysis has revealed that the immature form of BACE contains an N-terminal signal sequence (residues 1-21) followed by a large catalytic domain, a single transmembrane domain (residues 461-477), and a short cytoplasmic domain (residues 478-501). The signal sequence (1-21) is cleaved from the immature form by a signal peptidase located in the endoplasmic reticulum (ER), yielding the proBACE protein (Mr=75 kDa) which starts at residue 22. The proBACE protein is modified by cleavage of 24 N-terminal residues (aa 22-45), producing the mature BACE protein.
Specificity
Application
Immunohistochemistry on rat and monkey.
Optimal working dilutions must be determined by end user.
Target description
Analysis Note
Tested on human brain tissue lysate.
Legal Information
Not finding the right product?
Try our Product Selector Tool.
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service