493800
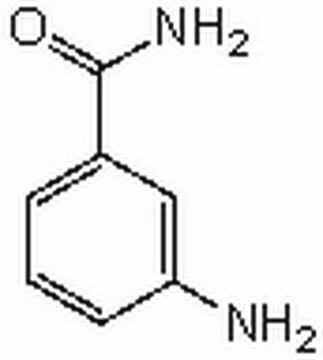
PARP Inhibitor VI, NU1025
The PARP Inhibitor VI, NU1025, also referenced under CAS 90417-38-2, controls the biological activity of PARP. This small molecule/inhibitor is primarily used for Cell Structure applications.
Synonym(s):
PARP Inhibitor VI, NU1025, 8-Hydroxy-2-methylquinazoline-4-one
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H8N2O2
CAS Number:
Molecular Weight:
176.17
MDL number:
UNSPSC Code:
12352200
Recommended Products
Quality Level
assay
≥98% (HPLC)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
protect from light
color
off-white
solubility
DMSO: 25 mg/mL
shipped in
ambient
storage temp.
−20°C
InChI
1S/C9H8N2O2/c1-5-10-8-6(9(13)11-5)3-2-4-7(8)12/h2-4,12H,1H3,(H,10,11,13)
InChI key
YJDAOHJWLUNFLX-UHFFFAOYSA-N
General description
A potent inhibitor poly(ADP-ribose) polymerase (PARP; IC50 = 0.40 µM). Has been shown to potentiate the cytotoxicity of the DNA-methylating agent MTIC [5-(3-N-methyltriazen-1-yl)-imidazole-4-carboxamide] and ionizing irradiation in murine L210 leukemia cells.
A potent poly(ADP-ribose) polymerase (PARP) inhibitor (IC50 = 400 nM) that potentiates the cytotoxicity of various DNA-active agents, including the DNA-methylating compound MTIC [5-(3-N-methyltriazen-1-yl)-imidazole-4-carboxamide], the DNA strand break-inducing drug temozolomide, topotecan, bleomycin, and ionizing radiation in murine L1210 leukemia cells, Chinese hamster ovary cells, and in a variety of human tumor cell lines.
Biochem/physiol Actions
Cell permeable: no
Primary Target
PARP
PARP
Product does not compete with ATP.
Reversible: no
Target IC50: 0.4 µM against poly(ADP-ribose) polymerase (PARP)
Packaging
Packaged under inert gas
Warning
Toxicity: Standard Handling (A)
Preparation Note
This solution can be further diluted 1:100 in tissue culture medium before use.
Reconstitution
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 6 months at -20°C.
Other Notes
Delaney, C.A., et al. 2000. Clin. Cancer Res.6, 2860.
Boulton, S., et al. 1999. Carcinogenesis20, 199.
Bowman, K.J., et al. 1998. Br. J. Cancer 78, 1269.
Griffin, R.J., et al. 1998. J. Med. Chem. 41, 5247.
Griffin, R.J., et al. 1996. Pharm. Sci. 2, 43.
Boulton, S., et al. 1995. Br. J. Cancer 72, 849.
Griffin, R.J., et al. 1995. Anticancer Drug Res. 10, 507.
Boulton, S., et al. 1999. Carcinogenesis20, 199.
Bowman, K.J., et al. 1998. Br. J. Cancer 78, 1269.
Griffin, R.J., et al. 1998. J. Med. Chem. 41, 5247.
Griffin, R.J., et al. 1996. Pharm. Sci. 2, 43.
Boulton, S., et al. 1995. Br. J. Cancer 72, 849.
Griffin, R.J., et al. 1995. Anticancer Drug Res. 10, 507.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service