W244910
Ethyl octanoate
natural, ≥98%, FCC, FG
Synonym(s):
Ethyl caprylate
About This Item
Halal
Kosher
natural
Recommended Products
grade
FG
Halal
Kosher
natural
agency
meets purity specifications of JECFA
reg. compliance
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 117
vapor pressure
0.02 mmHg ( 25 °C)
assay
≥98%
refractive index
n20/D 1.417 (lit.)
bp
206-208 °C (lit.)
mp
−48-−47 °C (lit.)
solubility
ethanol: soluble 1ml/4ml, clear, colorless (70% ethanol)
density
0.867 g/mL at 20 °C (lit.)
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
food allergen
no known allergens
organoleptic
banana; fruity; floral; pineapple
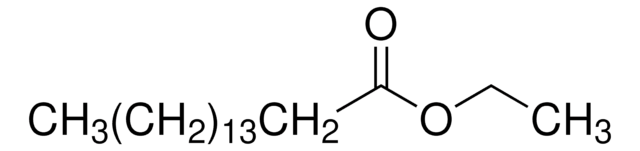
SMILES string
CCCCCCCC(=O)OCC
InChI
1S/C10H20O2/c1-3-5-6-7-8-9-10(11)12-4-2/h3-9H2,1-2H3
InChI key
YYZUSRORWSJGET-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
hcodes
pcodes
Hazard Classifications
Aquatic Chronic 2
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
167.0 °F - closed cup
flash_point_c
75 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W244910-100G | |
| W244910-100G-K | 4061837876783 |
| W244910-4KG-K | 4061837876806 |
| W244910-SAMPLE | |
| W244910-1KG | |
| W244910-1KG-K | 4061837876790 |
| W244910-4KG | |
| W244910-SAMPLE-K | 4061837876813 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service