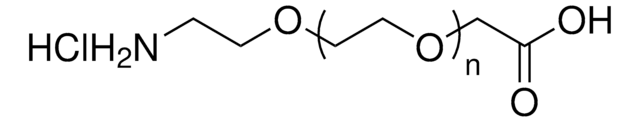
QBD10244
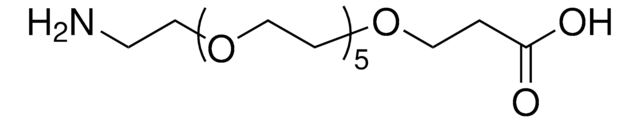
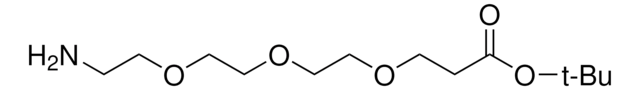
Amino-dPEG®4-acid
>95% (HPLC)
Synonym(s):
Amino-PEG-acid, Amino-PEG4-acid, CA(PEG)4, Carboxy-PEG4-amine, NH2-PEG4-COOH
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H23NO6
Molecular Weight:
265.30
MDL number:
UNSPSC Code:
12352106
NACRES:
NA.22
Recommended Products
assay
>95% (HPLC)
form
solid or viscous liquid
reaction suitability
reaction type: Pegylations
polymer architecture
shape: linear
functionality: heterobifunctional
shipped in
ambient
storage temp.
−20°C
Features and Benefits
Amino-dPEG4-acid has a primary amine and propionic acid terminating opposite ends of a 16-atoms long (18.0 Å) polyethylene glycol (PEG) spacer. The single molecular weight PEG spacer is discrete (Ð = 1). Moreover, it is highly hydrophilic, and it imparts hydrophilicity to conjugates that incorporate it. Amino-dPEG4-acid is useful in many different applications, including peptide synthesis, surface modification, dendrimer construction, and small molecule modification.
Legal Information
Products Protected under U.S. Patent #s 7,888,536 & 8,637,711 and European Patent #s 1,594,440 & 2,750,681
dPEG is a registered trademark of Quanta BioDesign
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lijun Wang et al.
Molecular pharmaceutics, 6(1), 231-245 (2008-12-11)
This report describes the synthesis of two cyclic RGD (Arg-Gly-Asp) conjugates, HYNIC-2PEG(4)-dimer (HYNIC = 6-hydrazinonicotinyl; 2PEG(4)-dimer = E[PEG(4)-c(RGDfK)](2); and PEG(4) = 15-amino-4,7,10,13-tetraoxapentadecanoic acid) and HYNIC-3PEG(4)-dimer (3PEG(4)-dimer = PEG(4)-E[PEG(4)-c(RGDfK)](2)), and evaluation of their (99m)Tc complexes [(99m)Tc(HYNIC-2PEG(4)-dimer)(tricine)(TPPTS)] ((99m)Tc-2PEG(4)-dimer: TPPTS = trisodium triphenylphosphine-3,3',3''-trisulfonate)
Kohei Sano et al.
Bioconjugate chemistry, 24(5), 811-816 (2013-04-23)
The ability to switch optical imaging probes from the quenched (off) to the active state (on) has greatly improved target to background ratios. The optimal activation efficiency of an optical probe depends on complete quenching before activation and complete dequenching
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service