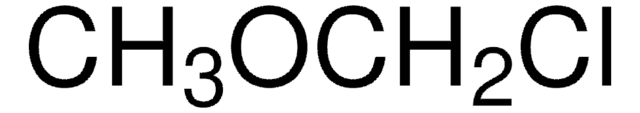C54007
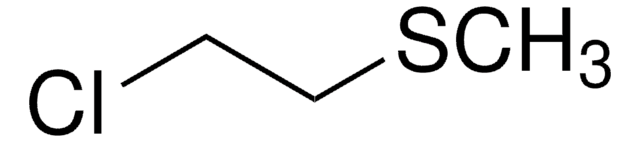
Chloromethyl methyl sulfide
95%
Synonym(s):
Chlorodimethyl sulfide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3SCH2Cl
CAS Number:
Molecular Weight:
96.58
Beilstein/REAXYS Number:
1730851
EC Number:
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
95%
form
liquid
refractive index
n20/D 1.498 (lit.)
bp
105 °C (lit.)
density
1.153 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CSCCl
InChI
1S/C2H5ClS/c1-4-2-3/h2H2,1H3
InChI key
JWMLCCRPDOIBAV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Methylthiomethylating reagent for carbonyl and aromatic compounds. Methylene transfer reagent for iron(II) mediated cyclopropanation.
Caution
May darken in storage.
signalword
Danger
hcodes
Hazard Classifications
Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
62.6 °F - closed cup
flash_point_c
17 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, 276-276 (1994)
Chemistry Letters (Jpn), 653-653 (1993)
Manuel Algarra et al.
The journal of physical chemistry. A, 124(10), 1911-1921 (2020-02-14)
UV photodecomposition of azidomethyl methyl sulfide (AMMS) yields a transient S-methylthiaziridine which rapidly evolves to S-methyl-N-sulfenylmethanimine at 10 K. This species was detected by infrared matrix isolation spectroscopy. The mechanism of the photoreaction of AMMS has been investigated by a
Organic Syntheses, 70, 177-177 (1992)
[Mutagenic activity of chlorodimethylsulfide and some of its aniline derivatives].
M Vito et al.
Bollettino della Societa italiana di biologia sperimentale, 61(6), 917-923 (1985-06-30)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service