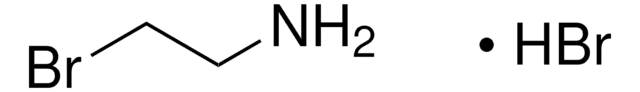
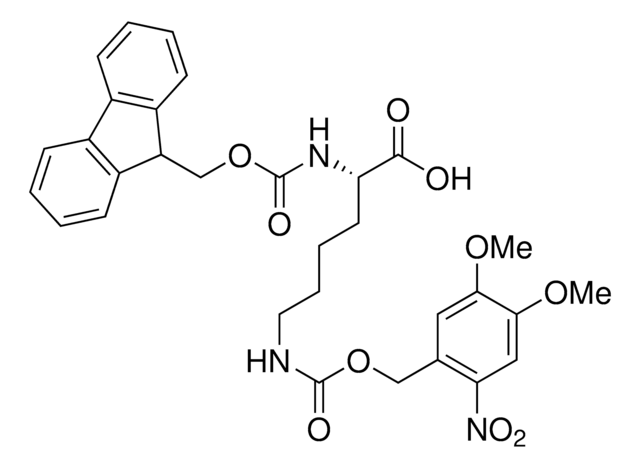
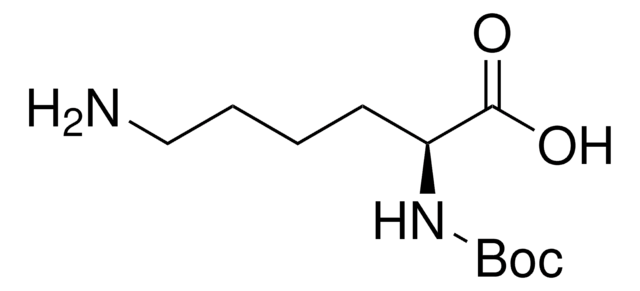
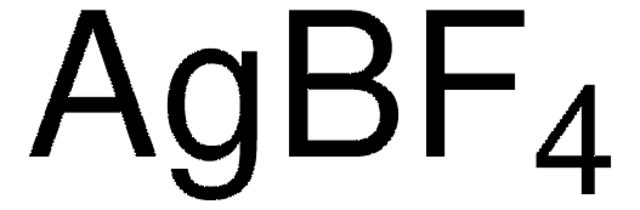913588
O-(2-Nitrobenzyl)-L-tyrosine hydrochloride
≥95%
Synonym(s):
(S)-2-Amino-3-(4-((2-nitrobenzyl)oxy)phenyl)propanoic acid hydrochloride, NBY, ONBY, Photo-controlled amino acid, Photocaged amino acid, Photocleavable tyrosine derivative
About This Item
Recommended Products
assay
≥95%
form
powder
availability
available only in USA
mp
205 °C (decomp.)
storage temp.
2-8°C
InChI
1S/C16H16N2O5.ClH/c17-14(16(19)20)9-11-5-7-13(8-6-11)23-10-12-3-1-2-4-15(12)18(21)22;/h1-8,14H,9-10,17H2,(H,19,20);1H/t14-;/m0./s1
InChI key
DRUCEARMIBXBOJ-UQKRIMTDSA-N
Application
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Time-resolved protein activation by proximal decaging in living systems
Crystal structure of a domain-swapped photoactivatable sfGFP variant provides evidence for GFP folding pathway
Light-control of the ultra-fast Gp41-1 split intein with preserved stability of a genetically encoded photo-caged amino acid in bacterial cells
Rapid and Inexpensive Evaluation of Nonstandard Amino Acid Incorporation in Escherichia coli
Related product
signalword
Danger
hcodes
Hazard Classifications
Self-react. C
Storage Class
5.2 - Organic peroxides and self-reacting hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![N-[2-(Fmoc-amino)-ethyl]-Gly-O-tBu hydrochloride ≥98.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/641/926/3fedc773-b21f-4419-afd5-87e20df0156a/640/3fedc773-b21f-4419-afd5-87e20df0156a.png)

![[2,2′-Bipyridine]-6-carboxylic acid hydrochloride](/deepweb/assets/sigmaaldrich/product/structures/130/786/b0e8142b-94b7-4abd-8a9a-0db3780a9f3d/640/b0e8142b-94b7-4abd-8a9a-0db3780a9f3d.png)