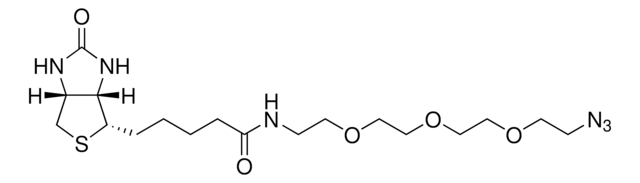
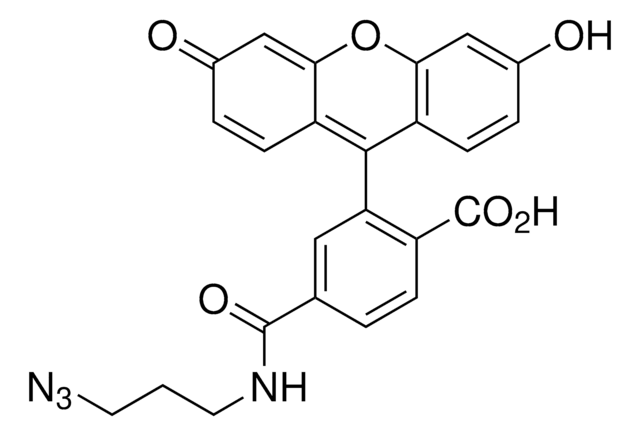
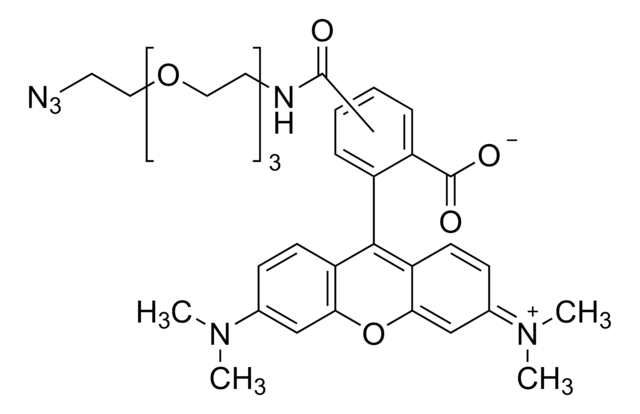
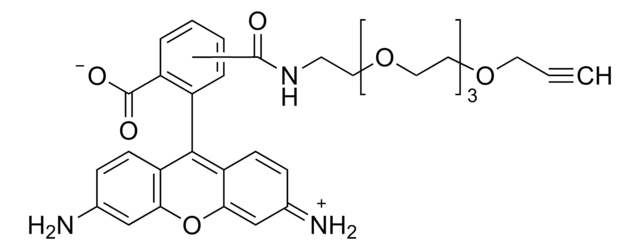
906328
BTTAA
≥95%
Synonym(s):
2-(4-((Bis((1-(tert-butyl)-1H-1,2,3-triazol-4-yl)methyl)amino)methyl)-1H-1,2,3-triazol-1-yl)acetic acid, Copper click-chemistry ligand, Water-soluble CuAAC ligand
About This Item
Recommended Products
assay
≥95%
form
solid
reaction suitability
reaction type: click chemistry
availability
available only in USA
storage temp.
2-8°C
Application
Other Notes
Fast, cell-compatible click chemistry with copper-chelating azides for biomolecular labeling
Metabolic labeling of fucosylated glycoproteins in Bacteroidales species
Increasing the Efficacy of Bioorthogonal Click Reactions for Bioconjugation: A Comparative Study
signalword
Danger
hcodes
Hazard Classifications
Self-react. C
Storage Class
5.2 - Organic peroxides and self-reacting hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)