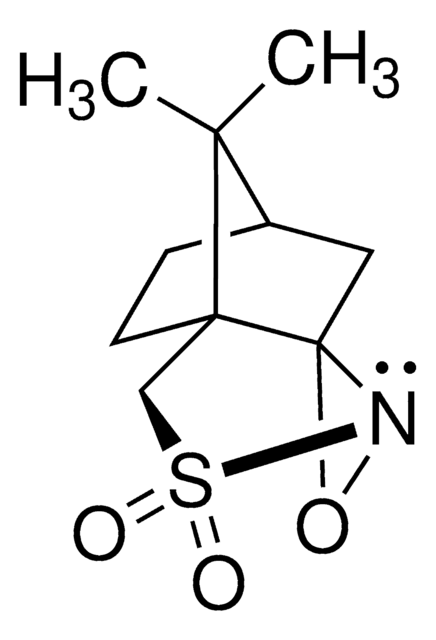
900624
Di-t-butyl oxaziridine
≥95%
Synonym(s):
Kurti oxaziridine
About This Item
Recommended Products
Quality Level
assay
≥95%
form
liquid
availability
available only in USA
refractive index
n/D 1.4453
density
0.90 g/mL
storage temp.
2-8°C
SMILES string
CC(C)(C)C1(NO1)C(C)(C)C
InChI
1S/C9H19NO/c1-7(2,3)9(10-11-9)8(4,5)6/h10H,1-6H3
Application
signalword
Danger
hcodes
Hazard Classifications
Self-react. C
Storage Class
5.2 - Organic peroxides and self-reacting hazardous materials
wgk_germany
WGK 3
flash_point_f
143.6 °F
flash_point_c
62 °C
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
Amines and their derivatives are ubiquitous substances since they make up the overwhelming majority of drug molecules, agrochemicals as well as many compounds that are produced by plants and living organisms (i.e., natural products).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service