693049
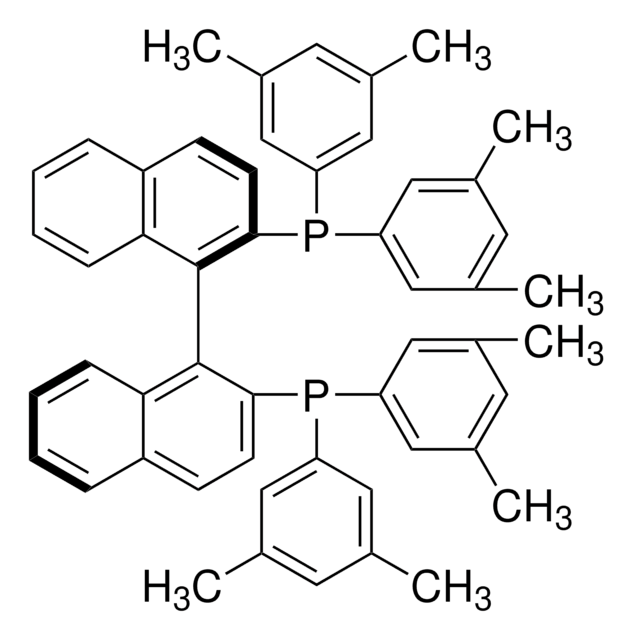
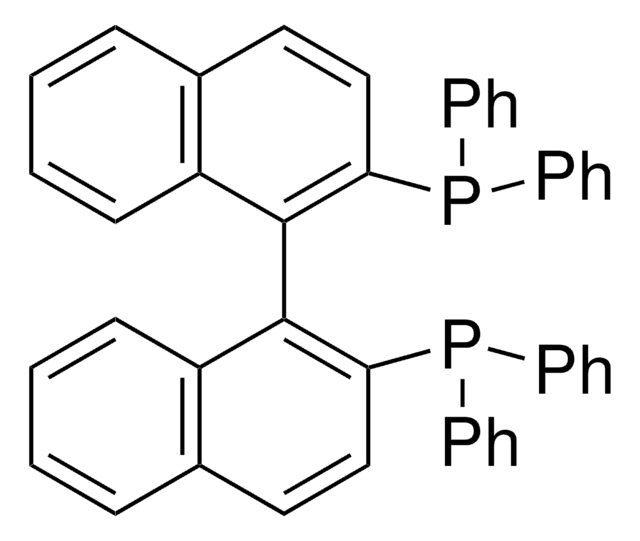
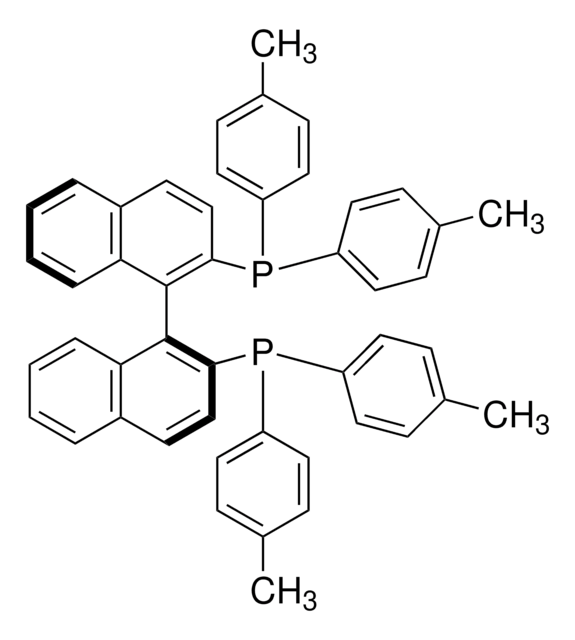
(R)-Tol-BINAP
Synonym(s):
(R)-(+)-2,2′-Bis(di-p-tolylphosphino)-1,1′-binaphthyl
About This Item
Recommended Products
form
solid
Quality Level
optical activity
[α]20/D +162°, c = 0.5 in benzene
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Cross Couplings
mp
254-258 °C
functional group
phosphine
SMILES string
Cc1ccc(cc1)P(c2ccc(C)cc2)c3ccc4ccccc4c3-c5c(ccc6ccccc56)P(c7ccc(C)cc7)c8ccc(C)cc8
InChI
1S/C48H40P2/c1-33-13-23-39(24-14-33)49(40-25-15-34(2)16-26-40)45-31-21-37-9-5-7-11-43(37)47(45)48-44-12-8-6-10-38(44)22-32-46(48)50(41-27-17-35(3)18-28-41)42-29-19-36(4)20-30-42/h5-32H,1-4H3
InChI key
IOPQYDKQISFMJI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Reactant serving as a precursor for:
- Catalysts used for reductive amination of ketones
- Rh(I)-catalyst for hydrogenation of acetamidoacrylic acid derivatives
- Chiral platinum catalysts for asymmetric Baeyer-Villiger oxidation of cyclic ketones
- CuI-Tol-BINAP catalysts for enantioselective Michael reactions of Grignard reagents to unsaturated esters
- BINAP Pt Dications for cation trapping
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts the ketones to esters and the cyclic ketones to lactones.
We present an article concerning BINAP/SEGPHOS® Ligands and Complexes.
Hydrogenation, Asymmetric Catalysis, Binap, SEGPHOS®, Aldol reaction, Alkenylation, Arylation, Mannich reaction, Fluorination, Michael addition, Hydrosilylation, Cycloaddition, Takasago
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service