680249
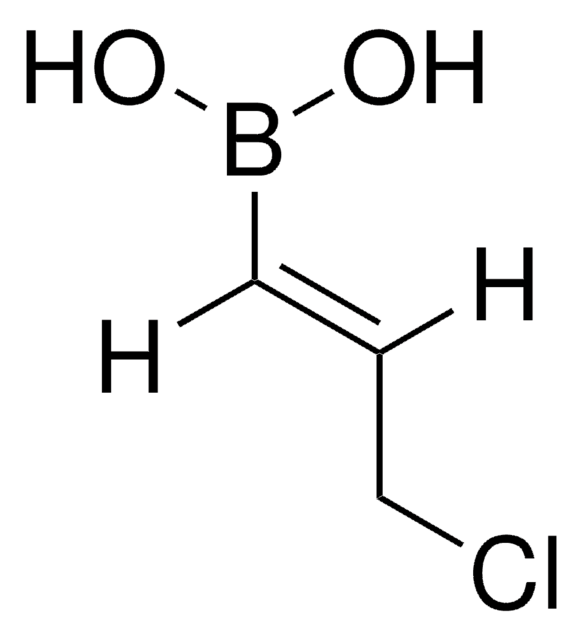
trans-2-Chloromethylvinylboronic acid pinacol ester
97%
Synonym(s):
(E)-2-(3-Chloro-1-propenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, E-2-Chloromethylvinylboronic acid pinacol ester
About This Item
Recommended Products
Quality Level
assay
97%
refractive index
n20/D 1.4603
bp
57-62 °C/0.3-0.4 mmHg
density
1.028 g/mL at 25 °C
functional group
chloro
SMILES string
CC1(C)OB(OC1(C)C)\C=C\CCl
InChI
1S/C9H16BClO2/c1-8(2)9(3,4)13-10(12-8)6-5-7-11/h5-6H,7H2,1-4H3/b6-5+
InChI key
HLBDNUSUVDDICF-AATRIKPKSA-N
Related Categories
Application
- Readout reagent that helps in providing qualitative and quantitative readouts in enzyme biomarker detection assays.
- Boronated enynes, which on Au(I) catalyzed cycloisomerization yield various dienyl boronates.
- α-Methyl-substituted boronates for the allylation of ketones to yield homoallylic tertiary alcohols.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
212.0 °F - closed cup
flash_point_c
100 °C - closed cup
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The synthesis of biaryl compounds via the Suzuki–Miyaura coupling reaction has become more commonplace now that many arylboronic acids are readily available.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)