663131
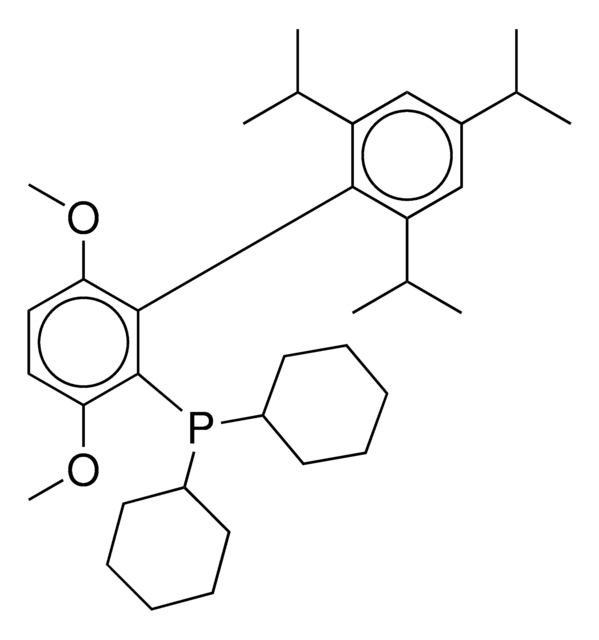
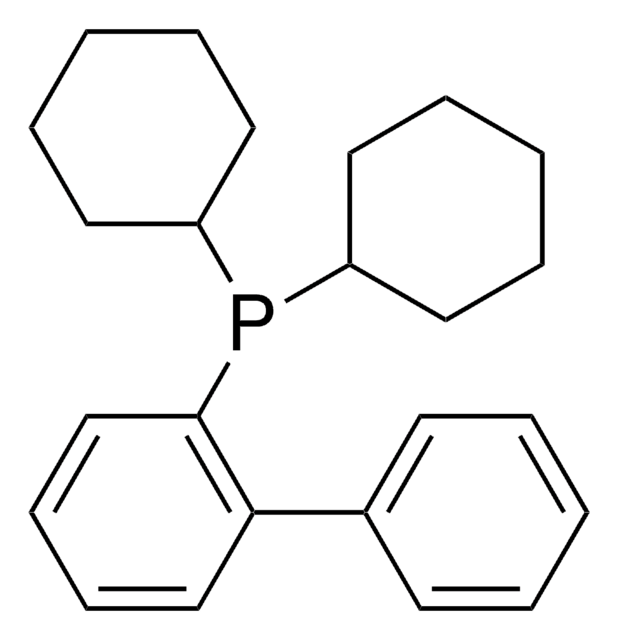
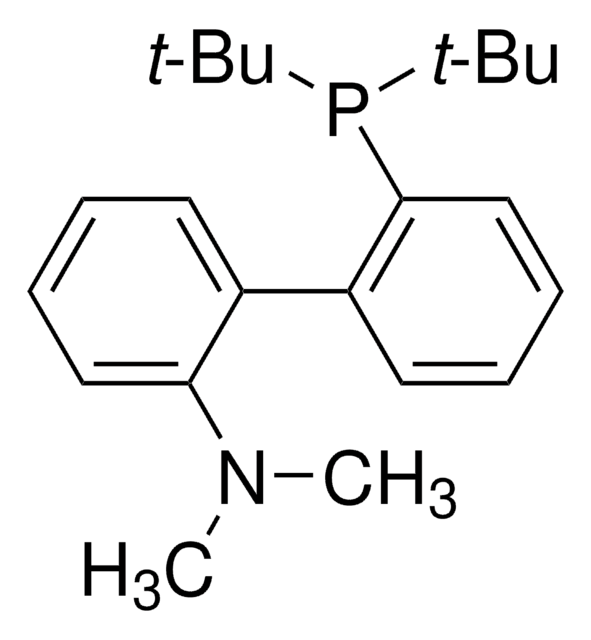
RuPhos
98%
Synonym(s):
2-Dicyclohexylphosphino-2′,6′-diisopropoxybiphenyl
About This Item
Recommended Products
assay
98%
form
solid
reaction suitability
reaction type: Cross Couplings
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
greener alternative product score
old score: 3
new score: 1
Find out more about DOZN™ Scoring
greener alternative product characteristics
Atom Economy
Design for Energy Efficiency
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurities
1-5% Bis(2′,6′-Diisopropoxybiphenyl)cyclohexylphosphine
mp
123-126 °C
functional group
phosphine
greener alternative category
SMILES string
CC(C)Oc1cccc(OC(C)C)c1-c2ccccc2P(C3CCCCC3)C4CCCCC4
InChI
1S/C30H43O2P/c1-22(2)31-27-19-13-20-28(32-23(3)4)30(27)26-18-11-12-21-29(26)33(24-14-7-5-8-15-24)25-16-9-6-10-17-25/h11-13,18-25H,5-10,14-17H2,1-4H3
InChI key
MXFYYFVVIIWKFE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
For small scale and high throughput uses, product is also available as ChemBeads (932205)
Legal Information
Related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service