All Photos(3)
About This Item
Empirical Formula (Hill Notation):
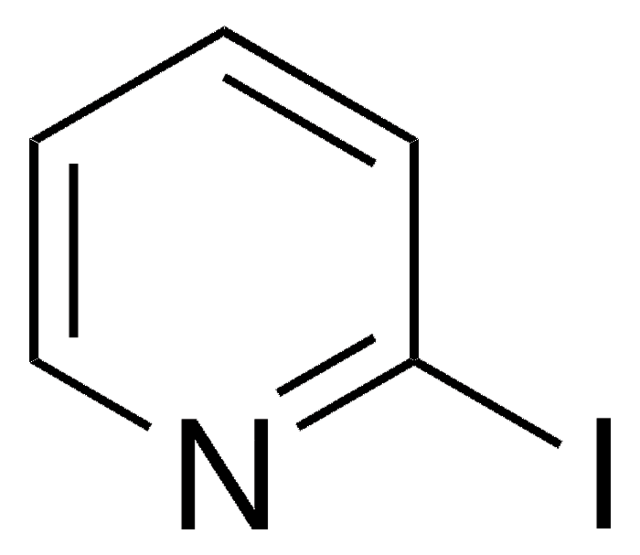
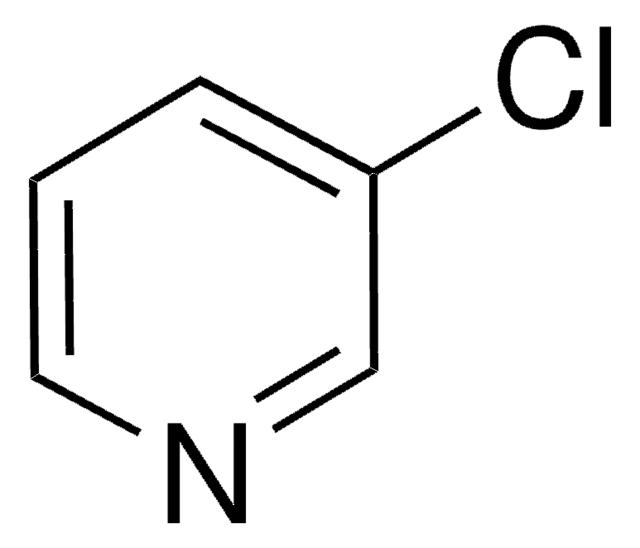
C5H4IN
CAS Number:
Molecular Weight:
205.00
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
mp
53-56 °C (lit.)
functional group
iodo
SMILES string
Ic1cccnc1
InChI
1S/C5H4IN/c6-5-2-1-3-7-4-5/h1-4H
InChI key
XDELKSRGBLWMBA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Iodopyridine is a heteroaryl halide. It undergoes microwave-assisted coupling with heterocyclic compounds (pyrazole, imidazole, pyrrole and indole) to afford the corresponding N-3-pyridinyl-substituted heterocyclic compounds.
Application
3-Iodopyridine may be used to synthesize following pyridine alkaloids:
- theonelladins C
- theonelladins D
- niphatesine C
- xestamine D
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
224.1 °F - closed cup
flash_point_c
106.7 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Microwave-assisted solvent-and ligand-free copper-catalysed cross-coupling between halopyridines and nitrogen nucleophiles.
Ernst, R. F.
Green Chemistry, 13(1), 42-45 (2011)
Synthesis of pyridine alkaloids via Pd-catalyzed coupling of 3-iodopyridine, 1, ?-dienes and nitrogen nucleophiles.
Larock RC and Wang Y.
Tetrahedron Letters, 43(1), 21-23 (2002)
Alina K Feldman et al.
Organic letters, 6(22), 3897-3899 (2004-10-22)
[reaction: see text] 1,4-Disubstituted 1,2,3-triazoles are obtained in excellent yields by a convenient one-pot procedure from a variety of readily available aromatic and aliphatic halides without isolation of potentially unstable organic azide intermediates.
Cameron C Bright et al.
Physical chemistry chemical physics : PCCP, 19(46), 31072-31084 (2017-11-21)
Small nitrogen containing heteroaromatics are fundamental building blocks for many biological molecules, including the DNA nucleotides. Pyridine, as a prototypical N-heteroaromatic, has been implicated in the chemical evolution of many extraterrestrial environments, including the atmosphere of Titan. This paper reports
Patrick W Fedick et al.
Journal of the American Society for Mass Spectrometry, 30(10), 2144-2151 (2019-08-09)
Suzuki cross-coupling is a widely performed reaction, typically using metal catalysts under heated conditions. Acceleration of the Suzuki cross-coupling reaction has been previously explored in microdroplets using desorption electrospray ionization mass spectrometry (DESI-MS). Building upon previous work, presented here is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service