479993
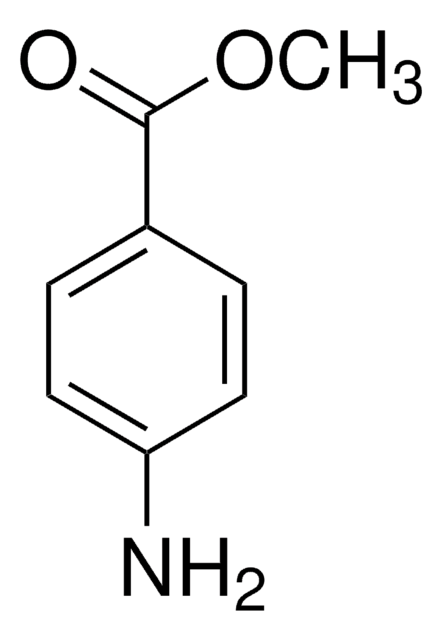
Methyl 4-(aminomethyl)benzoate hydrochloride
97%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NCH2C6H4CO2CH3·HCl
CAS Number:
Molecular Weight:
201.65
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
mp
243 °C (dec.) (lit.)
SMILES string
Cl.COC(=O)c1ccc(CN)cc1
InChI
1S/C9H11NO2.ClH/c1-12-9(11)8-4-2-7(6-10)3-5-8;/h2-5H,6,10H2,1H3;1H
InChI key
GIZCKBSSWNIUMZ-UHFFFAOYSA-N
General description
Methyl 4-(aminomethyl)benzoate hydrochloride is an amino acid ester hydrochloride. Its synthesis by esterification reaction has been reported. It plays a role during the preparation of a novel hepatitis C virus (HCV) helicase inhibitor.
Application
Methyl 4-(aminomethyl)benzoate hydrochloride may be used in the preparation of methyl 4-((3-butyl-3-phenylureido)methyl)benzoate.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sahar Kandil et al.
Bioorganic & medicinal chemistry letters, 19(11), 2935-2937 (2009-05-06)
Herein we report a successful application of a computer-aided design approach to identify a novel HCV helicase inhibitor. A de novo drug design methodology was used to generate an initial set of structures that could potentially bind to a putative
Joel A Bergman et al.
Journal of medicinal chemistry, 55(22), 9891-9899 (2012-09-27)
The incidence of malignant melanoma has dramatically increased in recent years thus requiring the need for improved therapeutic strategies. In our efforts to design selective histone deactylase inhibitors (HDACI), we discovered that the aryl urea 1 is a modestly potent
Xiaolin Ge et al.
Nature communications, 9(1), 2297-2297 (2018-06-14)
Synthetic polyelectrolytes, capable of fast transporting protons, represent a challenging target for membrane engineering in so many fields, for example, fuel cells, redox flow batteries, etc. Inspired by the fast advance in molecular machines, here we report a rotaxane based
Jiabo Li et al.
Molecules (Basel, Switzerland), 13(5), 1111-1119 (2008-06-19)
A series of amino acid methyl ester hydrochlorides were prepared in good to excellent yields by the room temperature reaction of amino acids with methanol in the presence of trimethylchlorosilane. This method is not only compatible with natural amino acids
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service