408484
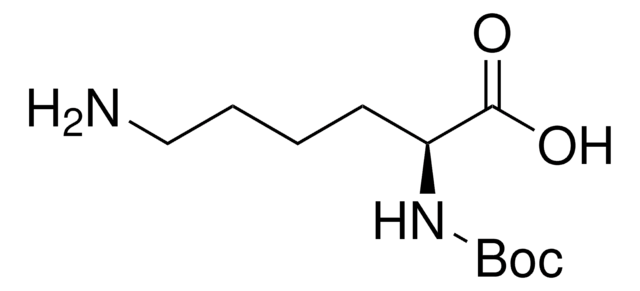
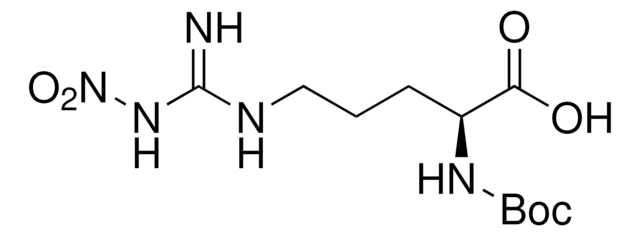
Boc-Arg-OH
for peptide synthesis
Synonym(s):
Nα-(tert-Butoxycarbonyl)-L-arginine, Nα-Boc-L-arginine
About This Item
Recommended Products
product name
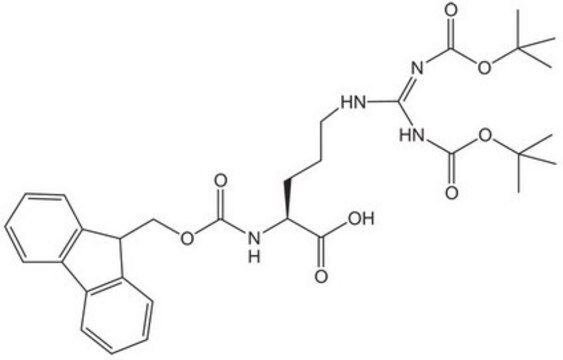
Boc-Arg-OH,
form
powder
Quality Level
optical activity
[α]20/D −6.5°, c = 1 in acetic acid
reaction suitability
reaction type: Boc solid-phase peptide synthesis
impurities
5-10% n-butanol
application(s)
peptide synthesis
SMILES string
CC(C)(C)OC(=O)N[C@@H](CCCNC(N)=N)C(O)=O
InChI
1S/C11H22N4O4/c1-11(2,3)19-10(18)15-7(8(16)17)5-4-6-14-9(12)13/h7H,4-6H2,1-3H3,(H,15,18)(H,16,17)(H4,12,13,14)/t7-/m0/s1
InChI key
HSQIYOPBCOPMSS-ZETCQYMHSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Studying the kinetics of tanning reactions: The study compares the color development kinetics in tanning reactions involving dihydroxyacetone with both free and Boc-protected basic amino acids, highlighting Boc-Arg-OH′s role in stabilizing reactive intermediates in cosmetic chemistry (Sun et al., 2022).
- Protonation states in peptide synthesis: This research examines the side-chain protonation states of a fluorescent arginine derivative, including Boc-Arg-OH, to optimize fluorescence in peptide synthesis for bioimaging applications (Marshall et al., 2019).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service