403482
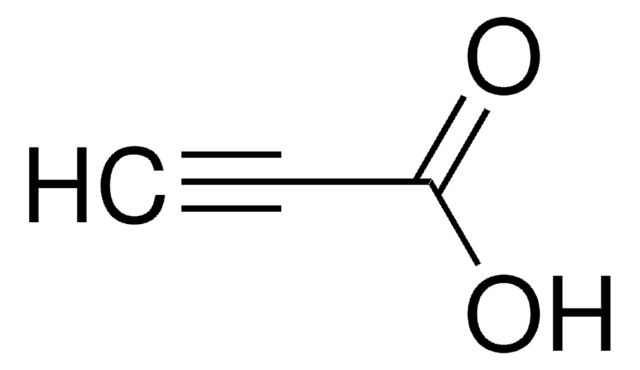
3-(Trimethylsilyl)propynoic acid
98%
Synonym(s):
3-(Trimethylsilyl)propiolic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)3SiC≡CCO2H
CAS Number:
Molecular Weight:
142.23
Beilstein/REAXYS Number:
1755674
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
assay:
98%
Recommended Products
Quality Level
assay
98%
bp
105-110 °C/10 mmHg (lit.)
mp
47-49 °C (lit.)
functional group
carboxylic acid
SMILES string
C[Si](C)(C)C#CC(O)=O
InChI
1S/C6H10O2Si/c1-9(2,3)5-4-6(7)8/h1-3H3,(H,7,8)
InChI key
IPEATTYBFBRNEB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-(Trimethylsilyl)propynoic acid (3-(Trimethylsilyl)propiolic acid) may be used as starting reagent for the synthesis of 3-trimethylsilylpropynamides.
3-(Trimethylsilyl)propynoic acid may be used in the regioselective preparation of 1,5-trisubstituted 1H-1,2,3-triazoles.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Steven J Coats et al.
Organic letters, 7(8), 1469-1472 (2005-04-09)
[reaction: see text] A regioselective method for the preparation of 1,5-trisubstituted 1H-1,2,3-triazoles via a 1,3-dipolar cycloaddition of 1-trimethylsilylacetylenes with organoazides is described. Immobilization of the azide on REM resin and subsequent cycloaddition afforded a 2 x 2 x 4 x
One-Pot Synthesis of 3-(Trimethylsilyl) propynamides.
Medvedeva AS, et al.
Russ. J. Org. Chem., 46(10), 1466-1470 (2010)
Yevgen Matviychuk et al.
Journal of magnetic resonance (San Diego, Calif. : 1997), 319, 106814-106814 (2020-09-20)
Low-cost, user-friendly benchtop NMR instruments are often touted as a "one-click" solution for data acquisition, however insufficient peak dispersion in their spectra often reduces the accuracy of quantification and requires user expertise with sophisticated processing tools. Our work aims to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service