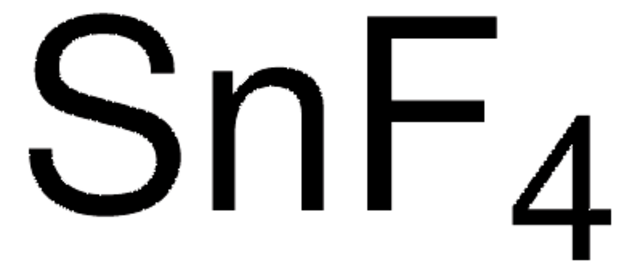334626
Tin(II) fluoride
99%
Synonym(s):
Difluorostannylene, Stannous fluoride, Tin bifluoride, Tin difluoride
About This Item
Recommended Products
Quality Level
assay
99%
reaction suitability
core: tin
reagent type: catalyst
density
4.57 g/mL at 25 °C (lit.)
SMILES string
F[SnH2]F
InChI
1S/2FH.Sn/h2*1H;/q;;+2/p-2
InChI key
ANOBYBYXJXCGBS-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Application
- As an inhibitor of Sn4+ in fabrication of formamidinium tin iodide (FASnI3) perovskite solar cells (PSCs) via SnF2–pyrazine complex formation.
- As a catalyst in glycerol ketalization, Knoevenagel condensation and aldol-type reactions.
- In the synthesis of α, β-epoxy ketone from α, α-dibromoacetophenone and aliphatic and aromatic aldehyde via oxidative addition of carbon-halogen bonds.
- In the stereocontrolled [3 + 3] , [3 + 4], [3 + 5] annulations reactions.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Met. Corr. 1 - Skin Irrit. 2
Storage Class
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service