All Photos(3)
About This Item
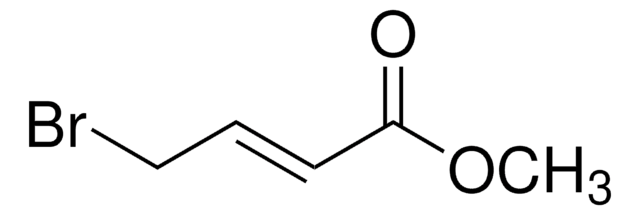
Linear Formula:
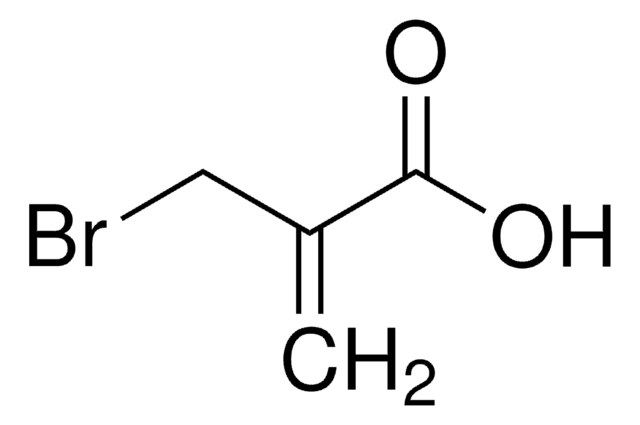
H2C=C(CH2Br)CO2CH3
CAS Number:
Molecular Weight:
179.01
Beilstein/REAXYS Number:
1852481
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
assay
97%
refractive index
n20/D 1.490 (lit.)
bp
35-37 °C/1.3 mmHg (lit.)
density
1.489 g/mL at 25 °C (lit.)
storage temp.
−20°C
SMILES string
COC(=O)C(=C)CBr
InChI
1S/C5H7BrO2/c1-4(3-6)5(7)8-2/h1,3H2,2H3
InChI key
CFTUQSLVERGMHL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Methyl (2-bromomethyl)acrylate (MBrMA) may be used as chain transfer agents in the emulsion polymerization of methyl methacrylate (MMA) and styrene. MBrMA can undergo nucleophilic substitution of carboxylic acid to form methyl 2-(acyloxymethyl)acrylates.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
172.4 °F - closed cup
flash_point_c
78 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bunichiro Yamada, Shuzo Aoki, Radical polymerization and copolymerization of methyl 2-(acyloxymethyl)acrylate as hindered 2-substituted acrylate
Kobatake S, et al.
Polymer, 36(2), 413-419 (1995)
Mukund P Sibi et al.
Tetrahedron, asymmetry, 17(4), 516-519 (2006-06-27)
We have investigated the effect of nitrogen protecting groups in radical addition trapping experiments leading to beta(2)-amino acids. Of the three N-protecting groups examined, the phthalimido group was optimal with respect to both yields and enantioselectivity. Additionally, radical additions to
Use of methyl 2-(bromomethyl) acrylate as a chain-transfer agent to yield functionalized macromonomers via conventional and living radical polymerizations.
Bon, SAF, et al.
Macromolecules, 33(16), 5819-5824 (2000)
Thomas Mendgen et al.
Bioorganic & medicinal chemistry letters, 20(19), 5757-5762 (2010-08-24)
The enzyme MurA performs an essential step in peptidoglycan biosynthesis and is therefore a target for the discovery of novel antibacterial compounds. We report here the inhibition of MurA by natural products from tulips (tulipalines and tuliposides), and the structure-activity
Fabrice Denes et al.
The Journal of organic chemistry, 72(2), 398-406 (2007-01-16)
Enantioenriched 3,4-disubstituted beta-prolines have been prepared with a high diastereocontrol through a carbometalation reaction or through a domino Michael addition/carbometalation reaction.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service