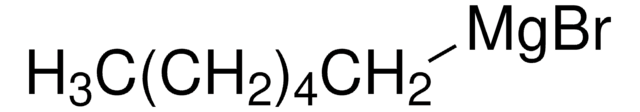290998
Pentylmagnesium bromide solution
2.0 M in diethyl ether
Synonym(s):
1-Pentylmagnesium bromide, n-Pentylmagnesium bromide, Amylmagnesium bromide, Bromopentylmagnesium
About This Item
Recommended Products
Quality Level
reaction suitability
reaction type: Grignard Reaction
concentration
2.0 M in diethyl ether
density
0.947 g/mL at 25 °C
SMILES string
CCCCC[Mg]Br
InChI
1S/C5H11.BrH.Mg/c1-3-5-4-2;;/h1,3-5H2,2H3;1H;/q;;+1/p-1
InChI key
ZXWGTPYWPZGZPT-UHFFFAOYSA-M
Application
- Alkanes via Ni or Cu-catalyzed cross-coupling reaction with alkyl fluorides.
- Hydroxypiperidinones via the addition-cyclization-deprotection process of aldimines.
It can also be used as a reagent to synthesize a key intermediate by olefin alkylation in the total synthesis of alkaloid, (+)-hyperaspine , and surface alkylation of H-terminated Si (111), by Si-C bond formation.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 1
target_organs
Central nervous system
supp_hazards
Storage Class
4.3 - Hazardous materials which set free flammable gases upon contact with water
wgk_germany
WGK 3
flash_point_f
-40.0 °F - closed cup
flash_point_c
-40 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service