All Photos(2)
About This Item
Empirical Formula (Hill Notation):
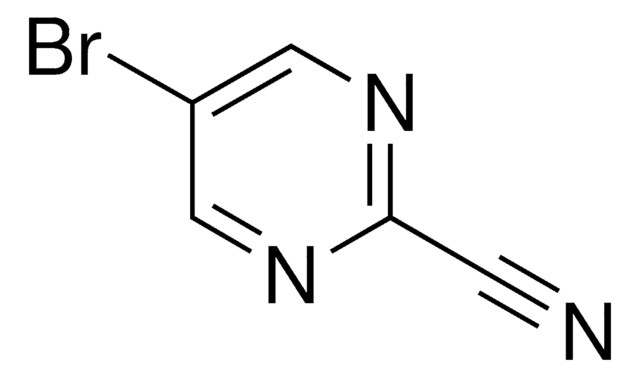
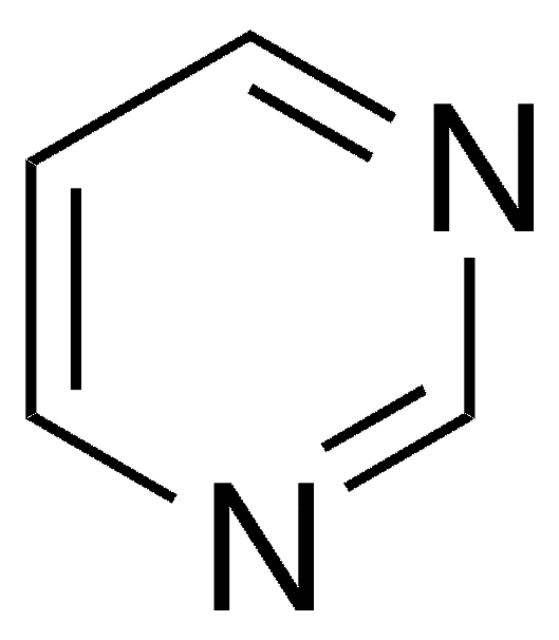
C4H3BrN2
CAS Number:
Molecular Weight:
158.98
Beilstein/REAXYS Number:
107326
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
form
solid
mp
67-73 °C (lit.)
functional group
bromo
SMILES string
Brc1cncnc1
InChI
1S/C4H3BrN2/c5-4-1-6-3-7-2-4/h1-3H
InChI key
GYCPLYCTMDTEPU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Rapid nucleophilic displacement reactions of 5-bromopyrimidine with nucleophiles under microwave irradiation has been studied. 5-Bromopyrimidine undergoes direct metallation with lithuium diisopropylamide to yield 4-lithio-5-bromopyrimidine.
Application
5-Bromopyrimidine was used in the synthesis of:
- N-heteroaryl substituted 9-arylcarbazolyl derivatives via palladium-catalyzed aerobic and ligand-free Suzuki reaction
- (5-(phenylethynyl)pyrimidine) via microwave assisted organic synthesis (MAOS) Sonogashira protocol
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The new NHS: restricting GPs' access to x ray services.
J Bahrami et al.
BMJ (Clinical research ed.), 302(6791), 1541-1541 (1991-06-22)
Direct metalation of pyrimidine. Synthesis of some 4-substituted pyrimidines.
Kress TJ.
The Journal of Organic Chemistry, 44(13), 2081-2082 (1979)
Efficient nucleophilic substitution reactions of pyrimidyl and pyrazyl halides with nucleophiles under focused microwave irradiation.
Cherng,Y-J.
Tetrahedron, 58(5), 887-890 (2002)
Xiaofeng Rao et al.
Organic & biomolecular chemistry, 10(39), 7875-7883 (2012-08-15)
A palladium-catalyzed aerobic and ligand-free Suzuki reaction in aqueous ethanol has been developed for the synthesis of N-heteroaryl substituted 9-arylcarbazolyl derivatives. A number of N-heteroaryl halides, namely 2-halogenated pyridines, 2-bromoquinoline, 5-bromopyrimidine and 2-chloropyrazine, were coupled with 4-(9H-carbazol-9-yl)phenylboronic acid (CPBA) or
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service