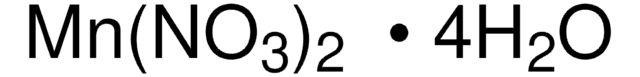203742
Manganese(II) nitrate hydrate
99.99% trace metals basis
Synonym(s):
Manganous dinitrate hydrate
About This Item
Recommended Products
grade
for analytical purposes
Quality Level
assay
99.99% trace metals basis
form
crystals and lumps
composition
Degree of hydration, 4-6
impurities
≤150.0 ppm Trace Metal Analysis
application(s)
battery manufacturing
storage temp.
2-8°C
SMILES string
O.[Mn++].[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/Mn.2NO3.H2O/c;2*2-1(3)4;/h;;;1H2/q+2;2*-1;
InChI key
HBTFASPVVFSRRI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- To fabricate tetragonal LiMn2O4 as a dual-functional pseudocapacitor-battery electrode via an in-situ ion-exchange method.
- As a safe, cost-effective, and efficient electrolyte for rapid energy storage applications like supercapacitors.
- As a precursor to prepare hybrid catalyst to enhance contaminants poisoning tolerance in solid oxide fuel cell cathodes.
- To prepare MnO2–CoO catalyst to fabricate opto-electronic humidity sensors.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
5.1B - Oxidizing hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Lithium-ion batteries represent a group of electrochemical devices used for electricity storage and have attracted a lot of attention in the past two decades due to their portability, rechargeability and low cost.
Thermoelectric Performance of Perovskite-type Oxide Materials
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 203742-100G | 4061838766304 |
| 203742-25G | 4061838766311 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service