180033
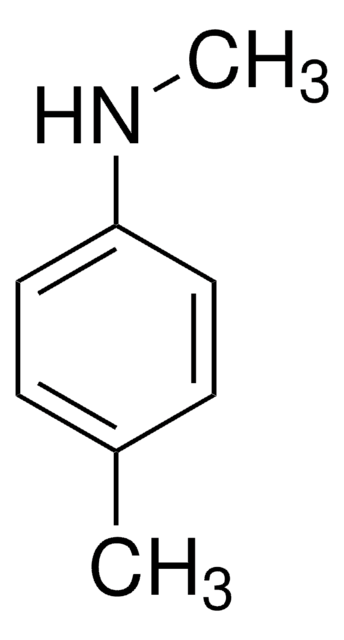
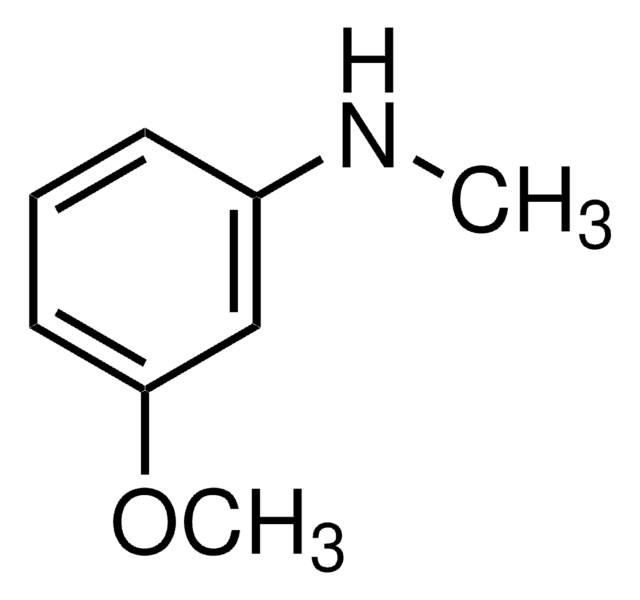
4-Methoxy-N-methylaniline
98%
Synonym(s):
N-Methyl-p-anisidine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3OC6H4NHCH3
CAS Number:
Molecular Weight:
137.18
Beilstein/REAXYS Number:
774640
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
solid
bp
135-136 °C/19 mmHg (lit.)
mp
33-36 °C (lit.)
functional group
amine
SMILES string
CNc1ccc(OC)cc1
InChI
1S/C8H11NO/c1-9-7-3-5-8(10-2)6-4-7/h3-6,9H,1-2H3
InChI key
JFXDIXYFXDOZIT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The allylation of N-methyl-p-anisidine by quaternary allylammonium cation was studied.
Application
4-Methoxy-N-methylaniline (N-Methyl-p-anisidine) was used to study the additive effects of amines.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Andrea Cappelli et al.
Bioorganic & medicinal chemistry, 16(6), 3428-3437 (2008-02-26)
The exploration of the structure-affinity relationships concerning a new class of peripheral benzodiazepine receptor (PBR) ligands related to alpidem has been pursued in order to evaluate the consistency of the structure-affinity relationships among different classes (and subclasses) of PBR ligands.
Takuto Nagano et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(37), 11578-11592 (2012-08-24)
The additive effects of amines were realized in the asymmetric hydrogenation of 2-phenylquinoxaline, and its derivatives, catalyzed by chiral cationic dinuclear triply halide-bridged iridium complexes [{Ir(H)[diphosphine]}(2)(μ-X)(3)]X (diphosphine = (S)-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl [(S)-BINAP], (S)-5,5'-bis(diphenylphosphino)-4,4'-bi-1,3-benzodioxole [(S)-SEGPHOS], (S)-5,5'-bis(diphenylphosphino)-2,2,2',2'-tetrafluoro-4,4'-bi-1,3-benzodioxole [(S)-DIFLUORPHOS]; X = Cl, Br, I) to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service