144916
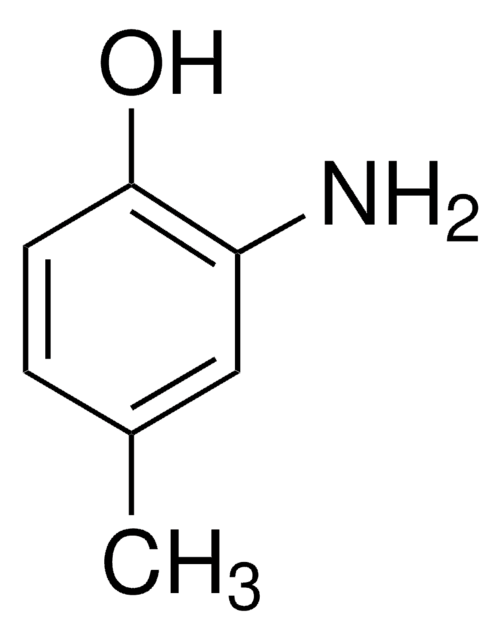
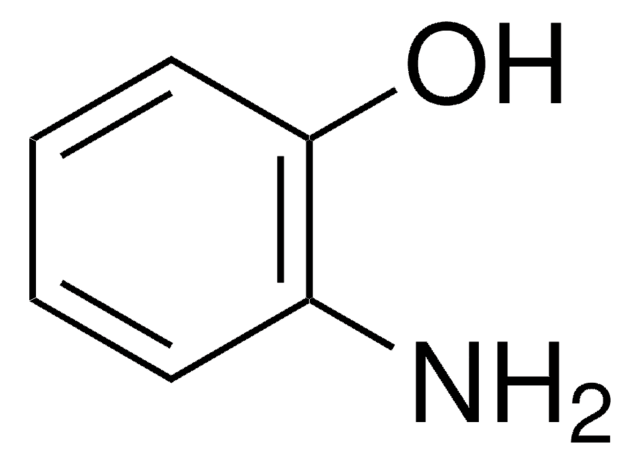
2-Amino-5-methylphenol
98%
Synonym(s):
2-Hydroxy-4-methylaniline, 4-Amino-3-hydroxytoluene, 6-Amino-m-cresol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NC6H3(CH3)OH
CAS Number:
Molecular Weight:
123.15
Beilstein/REAXYS Number:
386144
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
powder
mp
159-162 °C (lit.)
SMILES string
Cc1ccc(N)c(O)c1
InChI
1S/C7H9NO/c1-5-2-3-6(8)7(9)4-5/h2-4,9H,8H2,1H3
InChI key
HCPJEHJGFKWRFM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Amino-5-methylphenol reacts with bovine hemoglobin to form 2-amino-4,4α-dihydro-4α-7-dimethyl-3H-phenoxazine-3-one, which inhibits the proliferation of Poliovirus in Vero cells. It is converted to dihydrophenoxazinone by purified human hemoglobin.
Application
2-Amino-5-methylphenol was used in the synthesis of tridentate Schiff base ligand and novel non-metallocene catalysts with phenoxy-imine ligands.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Antiviral activity of 2-amino-4,4alpha-dihydro-4alpha-7-dimethyl-3H-phenoxazine-3-one on poliovirus.
Akiko Iwata et al.
The Tohoku journal of experimental medicine, 200(3), 161-165 (2003-10-03)
2-Amino-4,4alpha-dihydro-4alpha-7-dimethyl-3H-phenoxazine-3-one (Phx), which was produced by the reaction of bovine hemoglobin with 2-amino-5-methylphenol, inhibited the proliferation of poliovirus in Vero cells between 0.25 microg/ml and 2 microg/ml with maximal antiviral acitivity at 1 microg/ml. These results suggest that Phx may
Synthesis,structural characterization and catalytic activity study of Mn(II), Fe(III), Ni(II), Cu(II) and Zn(II) complexes of quinoxaline-2-carboxalidine-2-amino-5-methylphenol: Crystal structure of the nickel (II) complex.
Sebastian M, et al.
Polyhedron, 29(15), 3014-3020 (2010)
The investigation of novel non-metallocene catalysts with phenoxy-imine ligands for ethylene (co-) polymerization.
Zhang X, et al.
Polymer International, 62(3), 419-426 (2012)
A Tomoda et al.
Bioorganic & medicinal chemistry letters, 11(8), 1057-1058 (2001-05-01)
A simple and rapid preparation method for a novel antitumor agent, 2-amino-4,4a-dihydro-4a,7-dimethyl-3H-phenoxazine-3-one (Phx) was described. The procedure included (1) the reaction of bovine hemolysates with 2-amino-5-methylphenol, (2) one-shot denaturation of hemoglobin and proteins by methanol, and removal of the denatured
A Tomoda et al.
Biochimica et biophysica acta, 1117(3), 306-314 (1992-10-27)
We found that 2-amino-5-methylphenol was converted to the dihydrophenoxazinone with a reddish brown color by purified human hemoglobin, lysates of human erythrocytes, and human erythrocytes. The reddish brown compound was identified as 2-amino-4,4 alpha-dihydro-4 alpha,7-dimethyl-3H-phenoxazin-3-one by the measurement of NMR
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service