140066
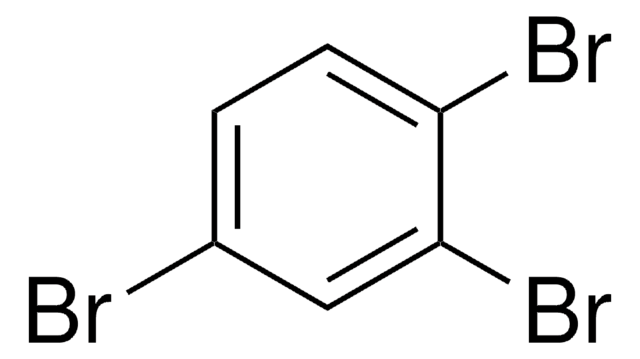
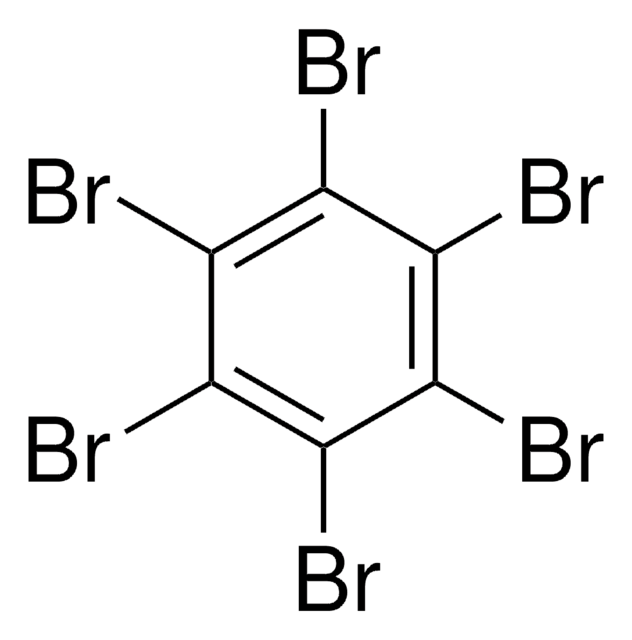
1,3,5-Tribromobenzene
98%
Synonym(s):
1,3,5-Tribromobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H3Br3
CAS Number:
Molecular Weight:
314.80
Beilstein/REAXYS Number:
1858917
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
powder
bp
271 °C (lit.)
mp
117-121 °C (lit.)
functional group
bromo
SMILES string
Brc1cc(Br)cc(Br)c1
InChI
1S/C6H3Br3/c7-4-1-5(8)3-6(9)2-4/h1-3H
InChI key
YWDUZLFWHVQCHY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
- 1,3,5-Tribromobenzene(TBB) acts as internal standard during derivatization step in determination of cyanide in human plasma and urine by gas chromatography-mass spectrometry.
- TBB forms molecular complexes of 1:2 stoichiometry with [60]-and [70] fullerenes.
- TBB reacts with perfluoroalkenylzinc reagent in the presence of Pd(Ph3)4 catalyst to yield a novel trifunctional monomer 1,3,5-tris(α, β, β-trifluorovinyl)benzene.
hcodes
Hazard Classifications
Aquatic Chronic 4
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Guojie Liu et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 877(27), 3054-3058 (2009-09-08)
Cyanide (CN) is a powerful poison and rapidly toxic agent. Because of its wide availability and high toxicity, quantification of CN in blood and urine is frequently required in clinical and forensic practice. We present a sensitive and less time
Lawrence A Ford et al.
Chemical communications (Cambridge, England), (20)(20), 2596-2597 (2003-11-05)
The reaction of the perfluoroalkenylzinc reagent, CF2=CFZnBr, with 1,3,5-tribromobenzene in the presence of a catalytic amount of Pd(Ph3)4 yielded a novel trifunctional monomer 1,3,5-tris(alpha,beta,beta-trifluorovinyl)benzene (1).
Kakali Datta et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 62(1-3), 66-70 (2005-11-01)
By UV-vis spectrophotometric method it has been shown that 1,3,5-tribromobenzene (TBB) forms molecular complexes of 1:2 stoichiometry with [60]- and [70]fullerenes. An isosbestic point could be detected in case of the [70]fullerene complex. The formation constant of the [60]fullerene complex
Y Suzuki et al.
Nucleic acids symposium series, (44)(44), 125-126 (2003-08-09)
We have designed a new type of a DNA dendrimer which has rigid branched structure. The branching molecule was prepared from 1,3,5-tribromobenzene. The dendrimer unit, in which three oligonucleotide-chains, two molecules of T15 and one molecule of A15, linked to
S Kage et al.
Journal of forensic sciences, 33(1), 217-222 (1988-01-01)
A sensitive analysis of sulfide in blood was established, using an extractive alkylation technique. Pentafluorobenzyl bromide was used as the alkylating agent, tetradecyldimethylbenzylammonium chloride as the phase-transfer catalyst, and potassium dihydrogenphosphate as the buffer to suppress the formation of sulfide.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service