All Photos(1)
About This Item
Linear Formula:
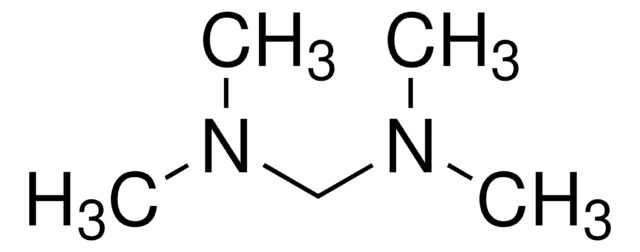
(C2H5)2NCH2N(C2H5)2
CAS Number:
Molecular Weight:
158.28
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
refractive index
n20/D 1.426 (lit.)
bp
166-169 °C (lit.)
density
0.811 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CCN(CC)CN(CC)CC
InChI
1S/C9H22N2/c1-5-10(6-2)9-11(7-3)8-4/h5-9H2,1-4H3
InChI key
UNEXJVCWJSHFNN-UHFFFAOYSA-N
Related Categories
Application
N,N,N′,N′-Tetraethylmethanediamine has been used to study the tertiary amine catalytic activities in the reaction of phenyl isocyanate with 1-butanol in toluene.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
107.6 °F - closed cup
flash_point_c
42 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tertiary Amine Catalysis of the Reaction of Phenyl Isocyanate with Alcohols.
BURKUS J
The Journal of Organic Chemistry, 26(3), 779-782 (1961)
Abdullah Alzahrani et al.
Organic & biomolecular chemistry, 16(22), 4108-4116 (2018-05-19)
The traditional thermal Mannich reaction is unsuitable for preparing polymerizable N-methylene amino substituted acrylamides and methacrylamides. Herein we provide a facile multi-gram high yield synthesis of these monomeric precursors to stimuli-responsive polymers by the addition of acrylamides and methacrylamides onto
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service