All Photos(1)
About This Item
Empirical Formula (Hill Notation):
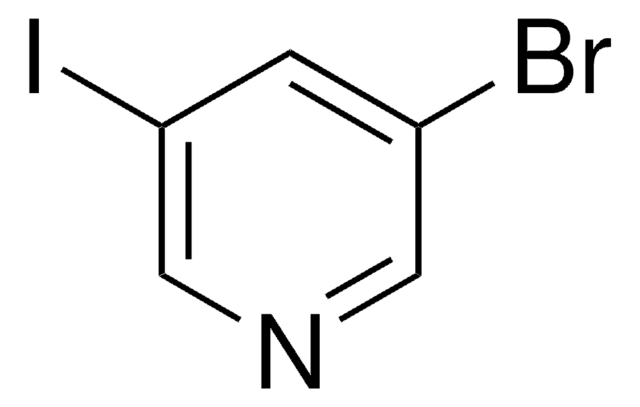
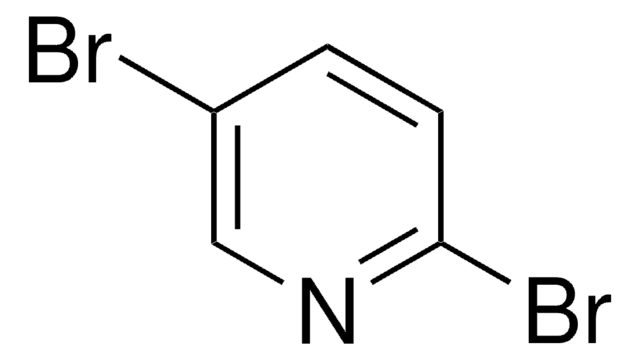
C5H3Br2N
CAS Number:
Molecular Weight:
236.89
Beilstein/REAXYS Number:
108477
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥98.0%
form
solid
mp
110-115 °C (lit.)
functional group
bromo
SMILES string
Brc1cncc(Br)c1
InChI
1S/C5H3Br2N/c6-4-1-5(7)3-8-2-4/h1-3H
InChI key
SOSPMXMEOFGPIM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3,5-Dibromopyridine undergoes lithiation with lithium diisopropylamide and on subsequent reaction with electrophiles yields 4-alkyl-3,5-dibromopyridines.
Application
3,5-Dibromopyridine was used in the synthesis of new Hg(II) complexes with halogen (chloro, bromo, iodo) and 3,5-disubstituted pyridine ligands.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of 4-alkyl-3, 5-dibromo-, 3-bromo-4, 5-dialkyl-and 3, 4, 5-trialkylpyridines via sequential metalation and metal-halogen exchange of 3, 5-dibromopyridine.
Gu YG and Bayburt EK.
Tetrahedron Letters, 37(15), 2565-2568 (1996)
Pyridine complexes of mercury (II) halides: Implications of a soft metal center for crystal engineering.
Hu C, et al.
CrystEngComm, 9(7), 603-610 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)