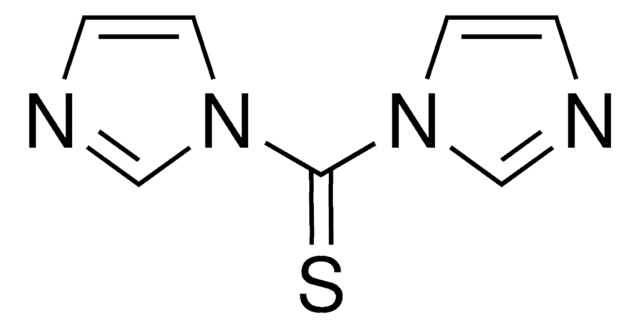
115533
CDI
≥90.0% (proton, NMR), for peptide synthesis
Synonym(s):
1,1′-Carbonyldiimidazole
About This Item
Recommended Products
product name
CDI, reagent grade
grade
reagent grade
Quality Level
assay
≥90.0% (proton, NMR)
form
solid
reaction suitability
reaction type: Carbonylations
greener alternative product characteristics
Designing Safer Chemicals
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
117-122 °C (lit.)
application(s)
peptide synthesis
greener alternative category
SMILES string
O=C(n1ccnc1)n2ccnc2
InChI
1S/C7H6N4O/c12-7(10-3-1-8-5-10)11-4-2-9-6-11/h1-6H
InChI key
PFKFTWBEEFSNDU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Repr. 1B - Skin Corr. 1B
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
N-Acylimidazoles were recognized in the early 1950s as reactive intermediates suitable for the acylation of amino compounds. The search for better coupling reagents than DCC led to the development of CDI (1,1’-carbonyldiimidazole) and related carbonylimidazoles.
Amide bonds are ubiquitous in both nature and industrial applications. They are vital to the structure and function of biological macromolecules and polymers. The importance of this functionality has resulted in numerous approaches to its formation, ranging from stoichiometric activation of carboxylic acids to more recent advances in catalytic amide bond formation.
Related Content
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)