Kluczowe dokumenty
T6258
Triosephosphate Isomerase from rabbit muscle
Type X, lyophilized powder, ≥3,500 units/mg protein
Synonim(y):
D-Glyceraldehyde-3-phosphate ketol-isomerase, TPI
About This Item
Polecane produkty
typ
Type X
Poziom jakości
Formularz
lyophilized powder
aktywność właściwa
≥3,500 units/mg protein
masa cząsteczkowa
calculated mol wt 53.2 kDa
skład
Protein, 60-90% biuret
obecność zanieczyszczeń
pyruvate kinase, lactic dehydrogenase, 3-phosphoglyceric phosphokinase, phosphoglucose isomerase, α-glycerophosphate dehydrogenase, aldolase and glyceraldehyde-3-phosphate dehydrogenase ≤0.01%
temp. przechowywania
−20°C
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
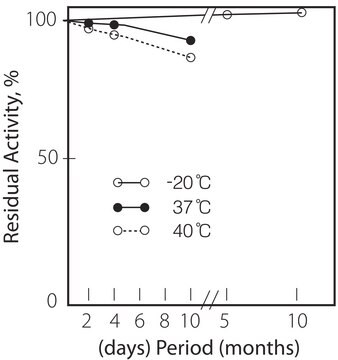
Działania biochem./fizjol.
Opakowanie
Definicja jednostki
Postać fizyczna
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej