Kluczowe dokumenty
T3580
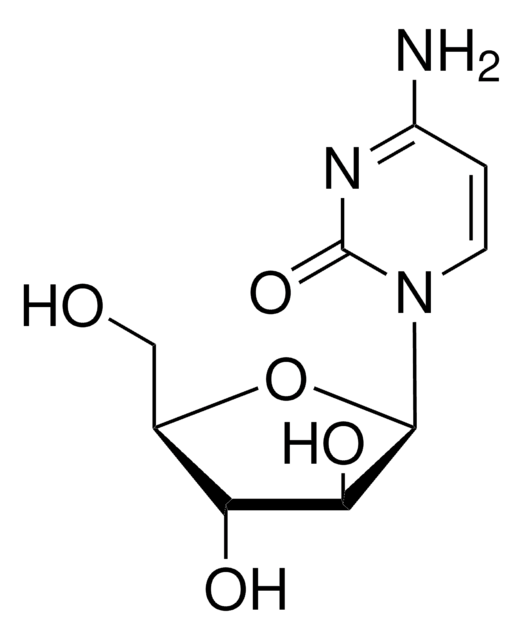
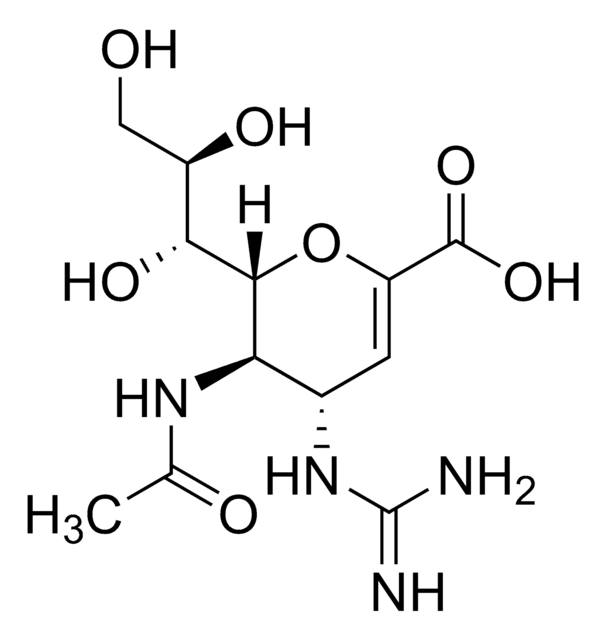
Toyocamycin
≥98% (HPLC), from Streptomyces rimosus
Synonim(y):
4-Aminopyrrolo[2,3-d]pyrimidine-5-carbonitrile 7-(β-D-ribofuranoside), 7-Deaza-7-cyanoadenosine, NSC 63701, NSC 99843, Neuro 000027, Unamycin B, Vengicide
About This Item
Polecane produkty
pochodzenie biologiczne
Streptomyces rimosus
Poziom jakości
Próba
≥98% (HPLC)
Formularz
solid
rozpuszczalność
DMSO: soluble 0.90-1.10 mg/mL, clear, colorless
H2O: moderately soluble
aqueous acid: moderately soluble
ethanol: moderately soluble
methanol: moderately soluble
spektrum działania antybiotyku
fungi
Tryb działania
DNA synthesis | interferes
temp. przechowywania
2-8°C
ciąg SMILES
Nc1ncnc2n(cc(C#N)c12)[C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O
InChI
1S/C12H13N5O4/c13-1-5-2-17(11-7(5)10(14)15-4-16-11)12-9(20)8(19)6(3-18)21-12/h2,4,6,8-9,12,18-20H,3H2,(H2,14,15,16)/t6-,8-,9-,12-/m1/s1
Klucz InChI
XOKJUSAYZUAMGJ-WOUKDFQISA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Działania biochem./fizjol.
Cechy i korzyści
Uwaga dotycząca przygotowania
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
We offers many products related to adenosine receptors for your research needs.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej






![PERK Inhibitor I, GSK2606414 GSK2606414 is a cell-permeable, highly potent inhibitor of EIF2AK3/PERK (IC₅₀ = 0.4 nM; [ATP] = 5 µM). Targets PERK in its inactive DFG conformation at the ATP-binding region.](/deepweb/assets/sigmaaldrich/product/structures/180/559/efa716dc-d5fe-4339-a6f0-0103084fc04a/640/efa716dc-d5fe-4339-a6f0-0103084fc04a.png)