Kluczowe dokumenty
T3405
3,3′,5,5′-Tetramethylbenzidine dihydrochloride
chromogenic, tablet
Synonim(y):
4,4′-Diamino-3,3′,5,5′-tetramethylbiphenyl dihydrochloride
About This Item
Polecane produkty
Nazwa produktu
3,3′,5,5′-Tetramethylbenzidine dihydrochloride, tablet, 1 mg substrate per tablet
Formularz
tablet
mp
≥300 °C
temp. przechowywania
2-8°C
ciąg SMILES
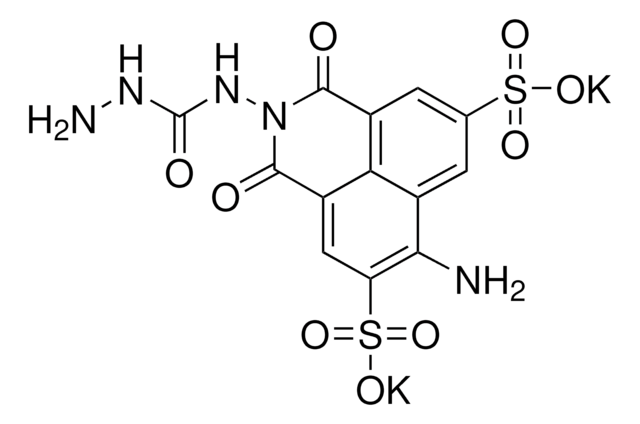
Cl[H].Cl[H].Cc1cc(cc(C)c1N)-c2cc(C)c(N)c(C)c2
InChI
1S/C16H20N2.2ClH/c1-9-5-13(6-10(2)15(9)17)14-7-11(3)16(18)12(4)8-14;;/h5-8H,17-18H2,1-4H3;2*1H
Klucz InChI
NYNRGZULARUZCC-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
- anti-mouse IgG (? specific) horseradish peroxidase-conjugated antibody in the ELISA of mice sera
- anti-mouse peroxidase antibody in the protein tyrosine phosphatase assay of naive and effector T cell lysate
- in enzymatic hydroperoxide (EH) assay and in prooxidants–antioxidants balance (PAB) assay of serum samples from diabetic patients
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Nitroblue Tetrazolium (NBT) is used with the alkaline phosphatase substrate 5-Bromo- 4-Chloro-3-Indolyl Phosphate (BCIP) in western blotting and immunohistological staining procedures. These substrate systems produce an insoluble NBT diformazan end product that is blue to purple in color and can be observed visually.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej