SML1524
(+)-JQ1
≥98% (HPLC), powder, BET bromodomain protein inhibitor
Synonim(y):
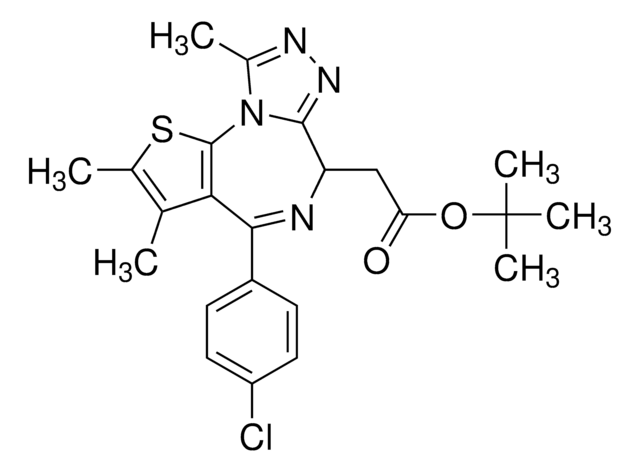
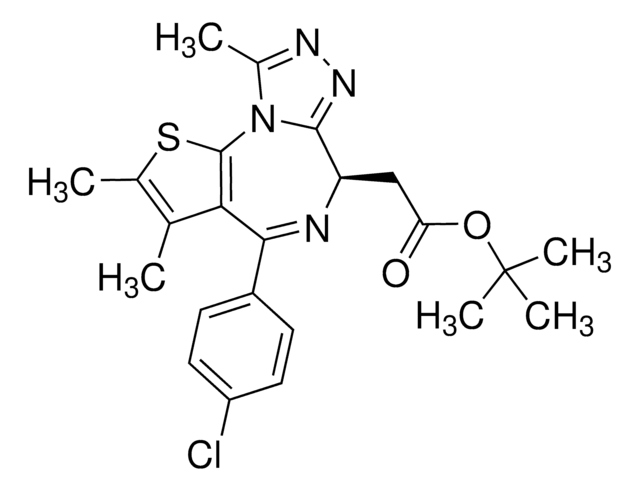
(S)-(+)-tert-Butyl 2-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate, 6H-Thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine-6-acetic acid, 4-(4-chlorophenyl)-2,3,9-trimethyl-, 1,1-dimethylethyl ester, (6S)-
About This Item
Polecane produkty
product name
(+)-JQ1, ≥98% (HPLC)
Poziom jakości
Próba
≥98% (HPLC)
Postać
powder
kolor
white to beige
rozpuszczalność
DMSO: 20 mg/mL, clear
temp. przechowywania
2-8°C
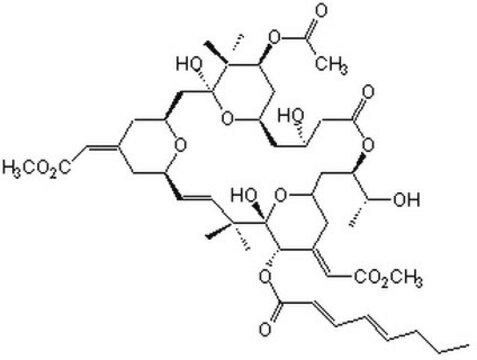
ciąg SMILES
O=C(OC(C)(C)C)C[C@H]1C2=NN=C(C)N2C(SC(C)=C3C)=C3C(C4=CC=C(Cl)C=C4)=N1
InChI
1S/C23H25ClN4O2S/c1-12-13(2)31-22-19(12)20(15-7-9-16(24)10-8-15)25-17(11-18(29)30-23(4,5)6)21-27-26-14(3)28(21)22/h7-10,17H,11H2,1-6H3/t17-/m0/s1
Klucz InChI
DNVXATUJJDPFDM-KRWDZBQOSA-N
Powiązane kategorie
Opis ogólny
Zastosowanie
Działania biochem./fizjol.
For characterization details of (+)-JQ1, please visit the JQ-1 probe summary on the Structural Genomics Consortium (SGC) website.
(-)-JQ1 is the negative control for the active enantiomer, (+)-JQ1. (-)-JQ1 is available from Sigma. To learn more about and purchase (-)-JQ1, click here.
To learn about other SGC chemical probes for epigenetic targets, visit sigma.com/sgc
Cechy i korzyści
Inne uwagi
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Powiązane treści
Poznaj analizę szlaków białkowych, w tym przesiewanie bibliotek chemicznych i modulowanie szlaków za pomocą małych cząsteczek.
Poznaj analizę szlaków białkowych, w tym przesiewanie bibliotek chemicznych i modulowanie szlaków za pomocą małych cząsteczek.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej