Key Documents
S2500
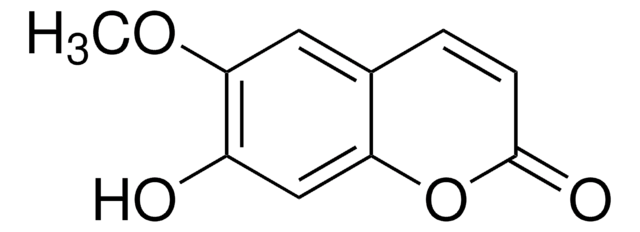
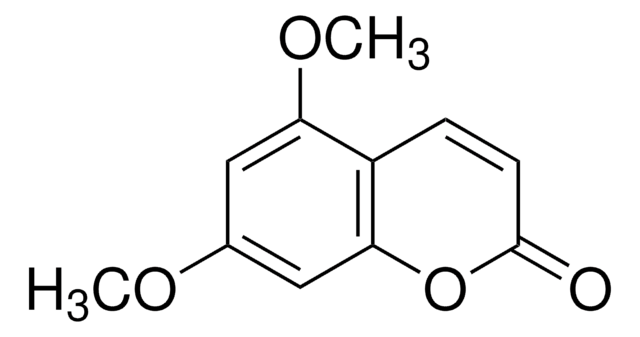
Scopoletin
≥99%
Synonim(y):
6-Methoxyumbelliferone, 6-Methylesculetin, 7-Hydroxy-6-methoxy-2H-1-benzopyran-2-one, 7-Hydroxy-6-methoxycoumarin, Buxuletin, Chrysatropic acid, Escopoletin, Esculetin-6-methyl ether, Gelseminic acid, Murrayetin
About This Item
Polecane produkty
Próba
≥99%
mp
203-205 °C (lit.)
ciąg SMILES
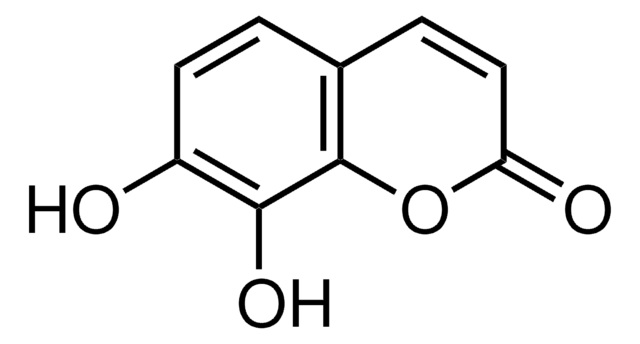
COc1cc2C=CC(=O)Oc2cc1O
InChI
1S/C10H8O4/c1-13-9-4-6-2-3-10(12)14-8(6)5-7(9)11/h2-5,11H,1H3
Klucz InChI
RODXRVNMMDRFIK-UHFFFAOYSA-N
informacje o genach
human ... ACHE(43)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
- Production of Polyphenolic Natural Products by Bract-Derived Tissue Cultures of Three Medicinal Tilia spp.: A Comparative Untargeted Metabolomics Study.: This study investigates the production of polyphenolic compounds, including scopoletin, in tissue cultures derived from the bracts of three medicinal Tilia species. The research highlights the potential of these cultures in producing valuable natural products (Szűcs et al., 2024).
Działania biochem./fizjol.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej