Kluczowe dokumenty
R109
Ro 15-4513
solid
Synonim(y):
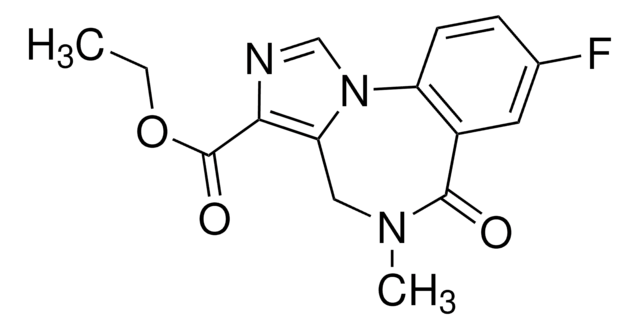
Ethyl 8-azido-6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate, Ro15-4513
About This Item
Polecane produkty
Formularz
solid
kolor
tan
rozpuszczalność
45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 0.8 mg/mL
H2O: insoluble
methanol: soluble
inicjator
Roche
ciąg SMILES
CCOC(=O)c1ncn-2c1CN(C)C(=O)c3cc(ccc-23)N=[N+]=[N-]
InChI
1S/C15H14N6O3/c1-3-24-15(23)13-12-7-20(2)14(22)10-6-9(18-19-16)4-5-11(10)21(12)8-17-13/h4-6,8H,3,7H2,1-2H3
Klucz InChI
CFSOJZTUTOQNIA-UHFFFAOYSA-N
informacje o genach
human ... GABRA1(2554) , GABRA2(2555) , GABRA3(2556) , GABRA5(2558) , GABRA6(2559)
rat ... Gabrg1(140674)
Zastosowanie
Działania biochem./fizjol.
Cechy i korzyści
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej