Key Documents
G1549
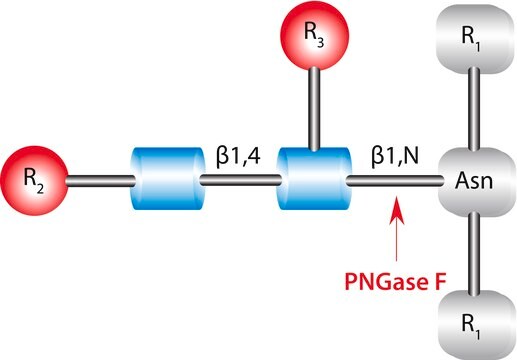
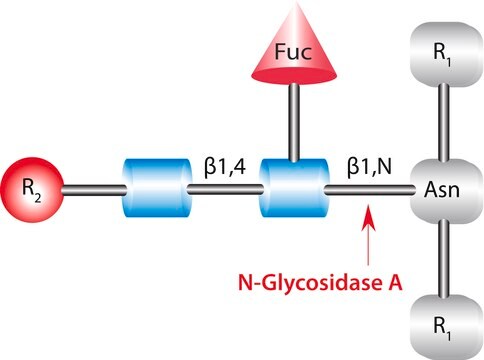
PNGase F from Elizabethkingia meningoseptica
ready-to-use solution, recombinant, expressed in E. coli
Synonim(y):
PNGase F from Elizabethkingia meningoseptica, N-Glycosidase F, PNGase F from Chryseobacterium meningosepticum, PNGase F from Flavobacterium meningosepticum, Peptide N-glycosidase
About This Item
Polecane produkty
rekombinowane
expressed in E. coli
Poziom jakości
białko sprzężone
(N-linked)
klasa czystości
Proteomics Grade
Postać
ready-to-use solution
aktywność właściwa
≥1000 U/mg
okres trwałości
≥1 yr at -20 °C
masa cząsteczkowa
~36 kDa
Warunki transportu
wet ice
temp. przechowywania
−20°C
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Zastosowanie
Działania biochem./fizjol.
Definicja jednostki
Postać fizyczna
Kod klasy składowania
10 - Combustible liquids
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej