Kluczowe dokumenty
D9568
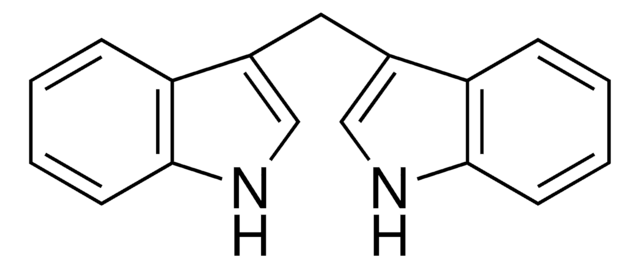
3,3′-Diindolylmethane
≥98% (HPLC)
Synonim(y):
3,3′-Bisindolylmethane, DIM
About This Item
Polecane produkty
Poziom jakości
Próba
≥98% (HPLC)
Formularz
powder
temp. przechowywania
2-8°C
ciąg SMILES
C(c1c[nH]c2ccccc12)c3c[nH]c4ccccc34
InChI
1S/C17H14N2/c1-3-7-16-14(5-1)12(10-18-16)9-13-11-19-17-8-4-2-6-15(13)17/h1-8,10-11,18-19H,9H2
Klucz InChI
VFTRKSBEFQDZKX-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
- 3,3′-Diindolylmethane inhibits Th17 cell differentiation via impairing IRF-7-mediated plasmacytoid dendritic cell activation in imiquimod-induced psoriasis mice.: The research indicates that 3,3′-Diindolylmethane can effectively inhibit Th17 cell differentiation, offering a potential therapeutic approach for treating psoriasis by targeting plasmacytoid dendritic cell pathways (Rasool et al., 2024).
- Protective effect of diindolylmethane-enriched dietary cabbage against doxorubicin-induced cardiotoxicity in mice.: This study highlights the cardioprotective effects of a diindolylmethane-enriched diet in mice, offering a dietary approach to mitigate the cardiotoxic effects of doxorubicin, a common chemotherapeutic agent (Natesh et al., 2024).
- Nanoformulated 3′-diindolylmethane modulates apoptosis, migration, and angiogenesis in breast cancer cells.: The investigation into nanoformulated 3′-diindolylmethane shows it significantly influences apoptosis, migration, and angiogenesis, suggesting its utility in targeted cancer therapies (Harakeh et al., 2024).
- Design, synthesis, and biological evaluation of 3,3′-diindolylmethane N-linked glycoconjugate as a leishmanial topoisomerase IB inhibitor with reduced cytotoxicity.: Research presents a synthesized glycoconjugate of 3,3′-Diindolylmethane as an effective inhibitor of leishmanial topoisomerase IB, demonstrating reduced cytotoxicity and potential as a therapeutic agent (Kour et al., 2023).
Działania biochem./fizjol.
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Aquatic Chronic 4 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organy docelowe
Respiratory system
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej