C6905
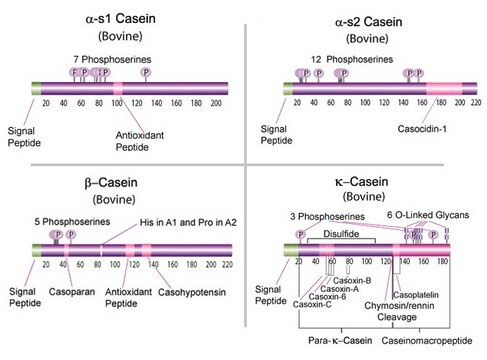
β-Casein from bovine milk
BioUltra, ≥98% (PAGE)
About This Item
Polecane produkty
pochodzenie biologiczne
bovine milk
Poziom jakości
linia produktu
BioUltra
Próba
≥98% (PAGE)
Formularz
essentially salt-free, lyophilized powder
metody
activity assay: suitable
temp. przechowywania
−20°C
ciąg SMILES
[P](=O)(OCC(NC(=O)C(NC(=O)C(N)Cc1ccccc1)CCC(=O)N)C(=O)NC(CCC(=O)O)C(=O)NC(CCC(=O)O)C(=O)NC(CCC(=O)N)C(=O)NC(CCC(=O)N)C(=O)NC(CCC(=O)N)C(=O)NC(C(O)C)C(=O)NC(CCC(=O)O)C(=O)NC(CC(=O)O)C(=O)NC(CCC(=O)O)C(=O)NC(CC(C)C)C(=O)NC(CCC(=O)N)C(=O)NC(CC(=O)O)C(=O)NC(C
InChI
1S/C81H125N22O39P/c1-36(2)31-50(76(132)94-43(15-24-57(87)108)71(127)101-52(34-64(120)121)78(134)98-49(81(137)138)11-7-8-30-82)99-72(128)47(19-28-61(114)115)95-77(133)51(33-63(118)119)100-73(129)48(20-29-62(116)117)97-80(136)65(37(3)104)103-75(131)44(16-25-58(88)109)92-68(124)42(14-23-56(86)107)90-67(123)41(13-22-55(85)106)91-69(125)45(17-26-59(110)111)93-70(126)46(18-27-60(112)113)96-79(135)53(35-142-143(139,140)141)102-74(130)40(12-21-54(84)105)89-66(122)39(83)32-38-9-5-4-6-10-38/h4-6,9-10,36-37,39-53,65,104H,7-8,11-35,82-83H2,1-3H3,(H2,84,105)(H2,85,106)(H2,86,107)(H2,87,108)(H2,88,109)(H,89,122)(H,90,123)(H,91,125)(H,92,124)(H,93,126)(H,94,132)(H,95,133)(H,96,135)(H,97,136)(H,98,134)(H,99,128)(H,100,129)(H,101,127)(H,102,130)(H,103,131)(H,110,111)(H,112,113)(H,114,115)(H,116,117)(H,118,119)(H,120,121)(H,137,138)(H2,139,140,141)
Klucz InChI
BECPQYXYKAMYBN-UHFFFAOYSA-N
informacje o genach
bovine ... CSN2(281099) , CSN3(281728)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
Działania biochem./fizjol.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 1
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
-casein basis (electrophoresis), lyophilized powder; β-Casein from bovine milk, BioUltra, ≥98% (PAGE)
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej