Kluczowe dokumenty
A5763
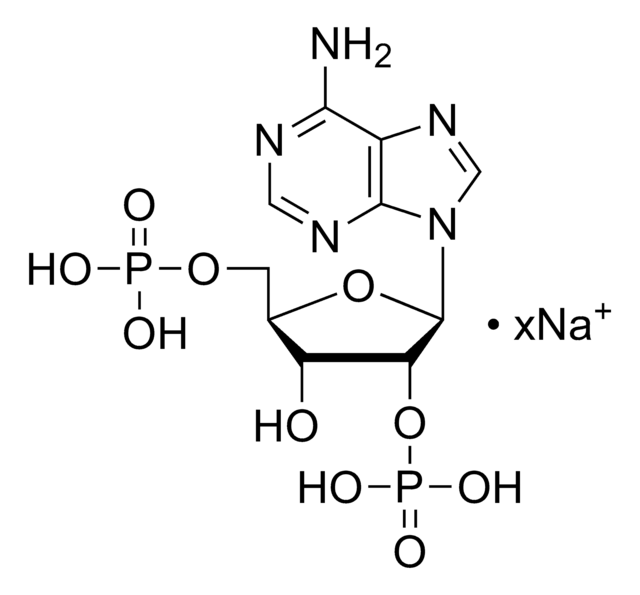
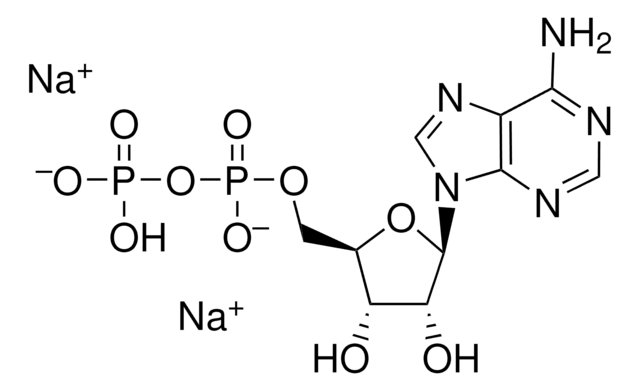
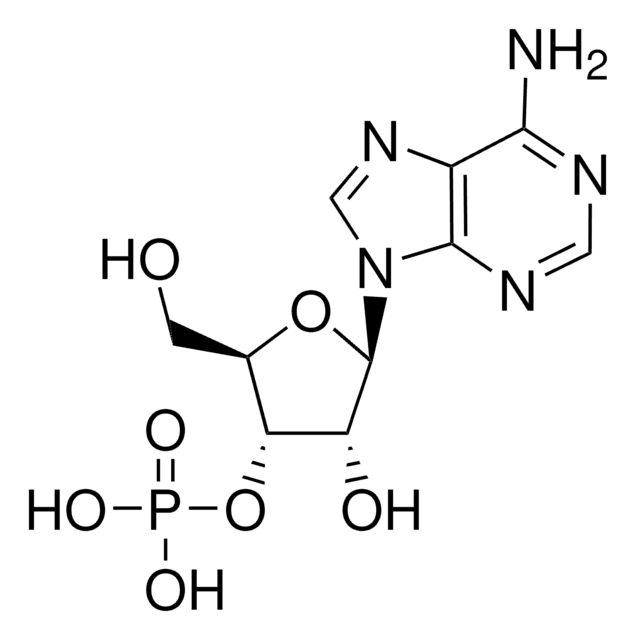
Adenosine 3′,5′-diphosphate disodium salt
≥96%
Synonim(y):
3′-phosphoadenosine 5′-phosphate, 3′-phosphorylated nucleotide, PAP, 3′-Phosphoadenosine 5′-phosphate
About This Item
Polecane produkty
pochodzenie biologiczne
synthetic (inorganic)
Poziom jakości
Próba
≥96%
Formularz
powder
rozpuszczalność
water: 25 mg/mL, clear, colorless to very faintly yellow
temp. przechowywania
−20°C
ciąg SMILES
[Na].Nc1ncnc2n(cnc12)C3OC(COP(O)(O)=O)C(OP(O)(O)=O)C3O
InChI
1S/C10H15N5O10P2.Na.H/c11-8-5-9(13-2-12-8)15(3-14-5)10-6(16)7(25-27(20,21)22)4(24-10)1-23-26(17,18)19;;/h2-4,6-7,10,16H,1H2,(H2,11,12,13)(H2,17,18,19)(H2,20,21,22);;
Klucz InChI
ISROZYFZEAVMSP-UHFFFAOYSA-N
Powiązane kategorie
Opis ogólny
Zastosowanie
- to spot sample on cellulose high-performance thin-layer chromatography (HPTLC) plates in two-dimensional thin layer chromatography
- in enzyme activity assay to study the activities of HOS2/FIERY1 wild type
- hos2 mutant and fiery1?2 mutant protein against 3′-phosphoadenosine 5′-phosphate (PAP)
- as a standard for the quantification of phosphoadenosines
Działania biochem./fizjol.
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organy docelowe
Respiratory system
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej