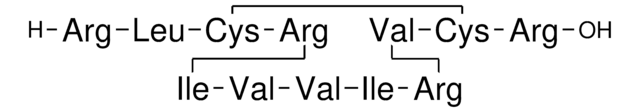C5241
Cinnamycin
from Streptomyces cinnamoneus, ≥95% (HPLC)
Synonim(y):
Lanthiopeptin, NSC-71936, Ro 09-0198
About This Item
Polecane produkty
pochodzenie biologiczne
Streptomyces cinnamoneus
Poziom jakości
Próba
≥95% (HPLC)
Formularz
solid
rozpuszczalność
DMSO: 10 mg/mL
acetonitrile: water (1:1): 5 mg/mL (requires heating)
spektrum działania antybiotyku
fungi
Tryb działania
cell membrane | interferes
temp. przechowywania
2-8°C
ciąg SMILES
S1[C@@H](C2NC(=O)[C@@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H]3NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H]5NC(=O)[C@@H](NC(=O)[C@H]7N(CCC7)C(=O)CNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](C1)N)CCCN\C(=N/[H])\N)CCC(=O)N)CS
InChI
1S/C89H125N25O25S3/c1-43(2)66-84(133)109-59-41-140-40-58-79(128)108-60-42-142-45(4)68(86(135)105-55(75(124)110-66)34-48-22-12-7-13-23-48)111-76(125)54(33-47-20-10-6-11-21-47)104-82(131)61-26-17-31-114(61)65(118)38-98-72(121)53(32-46-18-8-5-9-19-46)103-78(127)57(106-80(60)129)36-95-29-15-14-24-52(87(136)137)102-85(134)67(112-77(126)56(35-63(92)116)99-64(117)37-97-83(132)69(113-81(59)130)70(119)88(138)139)44(3)141-39-49(90)71(120)100-50(25-16-30-96-89(93)94)73(122)101-51(74(123)107-58)27-28-62(91)115/h5-13,18-23,43-45,49-61,66-70,95,119H,14-17,24-42,90H2,1-4H3,(H2,91,115)(H2,92,116)(H,97,132)(H,98,121)(H,99,117)(H,100,120)(H,101,122)(H,102,134)(H,103,127)(H,104,131)(H,105,135)(H,106,129)(H,107,123)(H,108,128)(H,109,133)(H,110,124)(H,111,125)(H,112,126)(H,113,130)(H,136,137)(H,138,139)(H4,93,94,96)/t44-,45?,49+,50+,51+,52+,53+,54+,55+,56+,57+,58+,59+,60+,61+,66+,67?,68+,69+,70-/m1/s1
Klucz InChI
QJDWKBINWOWJNZ-IDGBIKHQSA-N
Powiązane kategorie
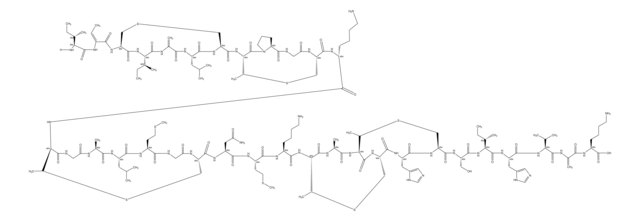
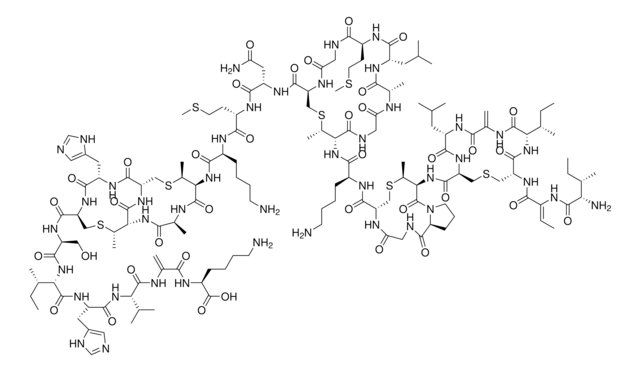
Amino Acid Sequence
Opis ogólny
Zastosowanie
Działania biochem./fizjol.
Cinnamycin, like other lantibiotics, was also reported to inhibit phospholipase A2 (PLA2). It was suggested as an alternative treatment for atherosclerosis through its ability to inhibit PLA2 by binding to its substrate PE. Moreover, Cinnamycin was found to inhibit Herpes simplex virus (HSV-1) activity.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Syntetyzowane rybosomalnie peptydy przeciwdrobnoustrojowe są obiecującym przedmiotem badań nad antybiotykami w obliczu oporności bakterii i pojawiających się chorób zakaźnych.
With bacterial resistance and emerging infectious diseases becoming potential threats to humans, ribosomally synthesized antimicrobial peptides have become a promising focus area in antibiotic research.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej