Kluczowe dokumenty
53404
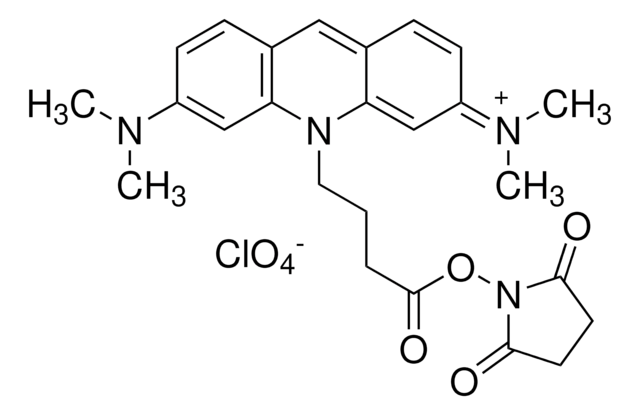
Atto 465 NHS ester
BioReagent, suitable for fluorescence, ≥90% (HPLC)
About This Item
Polecane produkty
linia produktu
BioReagent
Próba
≥90% (HPLC)
≥90% (degree of coupling)
producent / nazwa handlowa
ATTO-TEC GmbH
λ
in acetonitrile (with 0.1% acetic acid)
absorpcja UV
λ: 454-460 nm Amax
przydatność
suitable for fluorescence
temp. przechowywania
−20°C
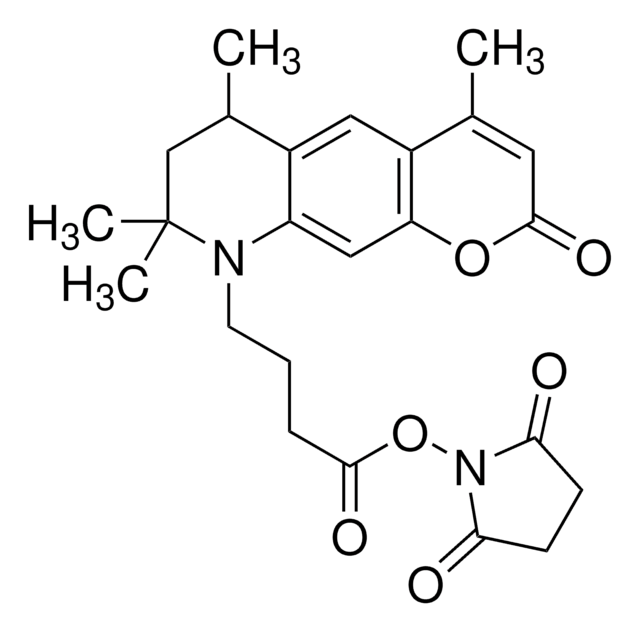
ciąg SMILES
OCl(=O)(=O)=O.Nc1ccc2C=C3C=CC(=N)C=C3N(CCCC(=O)ON4C(=O)CCC4=O)c2c1
InChI
1S/C21H20N4O4.ClHO4/c22-15-5-3-13-10-14-4-6-16(23)12-18(14)24(17(13)11-15)9-1-2-21(28)29-25-19(26)7-8-20(25)27;2-1(3,4)5/h3-6,10-12,22H,1-2,7-9,23H2;(H,2,3,4,5)
Klucz InChI
NROCSLJOXCQUHR-UHFFFAOYSA-N
Zastosowanie
Informacje prawne
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Fluorescent Labeling of Peptides
Pochodne chromogeniczne i fluorogeniczne są nieocenionymi narzędziami w biochemii, mającymi liczne zastosowania w enzymologii, chemii białek, immunologii i histochemii.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej