PHR1896
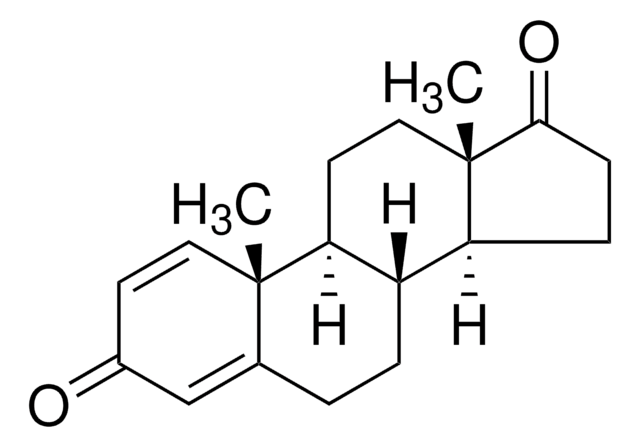
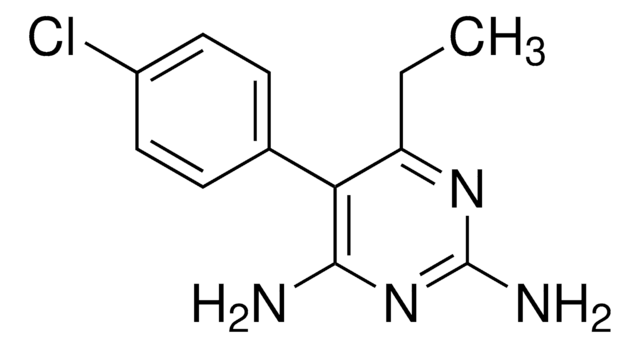
Związek D związany z eksemestanem
Pharmaceutical Secondary Standard; Certified Reference Material
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Kod UNSPSC:
41116107
NACRES:
NA.24
Polecane produkty
klasa czystości
certified reference material
pharmaceutical secondary standard
Poziom jakości
rodzina API
exemestane
Certyfikat analizy
current certificate can be downloaded
opakowanie
pkg of 30 mg
Zastosowanie
pharmaceutical
Format
neat
temp. przechowywania
2-8°C
Opis ogólny
Exemestane Related Compound D jest zanieczyszczeniem steroidowego leku przeciwnowotworowego eksemestanu. Eksemestan należy do klasy antyestrogenów znanych jako inhibitory aromatazy i jest powszechnie stosowany w leczeniu raka piersi.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Zastosowanie
Eksemestan może być stosowany jako farmaceutyczny wzorzec odniesienia do oznaczania analitu w lekach luzem i preparatach farmaceutycznych metodą chromatografii.
Te wzorce wtórne są kwalifikowane jako certyfikowane materiały odniesienia. Są one odpowiednie do stosowania w wielu aplikacjach analitycznych, w tym między innymi w testach uwalniania farmaceutycznego, opracowywaniu metod farmaceutycznych do analiz jakościowych i ilościowych, testach kontroli jakości żywności i napojów oraz innych wymaganiach kalibracyjnych.
Komentarz do analizy
Te drugorzędne standardy oferują możliwość wielokrotnego śledzenia do podstawowych standardów USP, EP i BP, gdzie są one dostępne.
Inne uwagi
Ten certyfikowany materiał referencyjny (CRM) jest produkowany i certyfikowany zgodnie z normami ISO 17034 i ISO/IEC 17025. Wszystkie informacje dotyczące stosowania tego CRM można znaleźć w certyfikacie analizy.
Przypis
To see an example of a Certificate of Analysis for this material enter LRAB0340 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
Ta strona może zawierać tekst przetłumaczony maszynowo.
produkt powiązany
Numer produktu
Opis
Cennik
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumenty section.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Analytical method validation for HPLC assay of oral anticancer drug exemestane
Yavuz B
Journal of Pharmaceutical Sciences, 32(1), 15-15 (2007)
Exemestane: a review of its clinical efficacy and safety
L?nning PE
Breast (Edinburgh, Scotland), 10(3), 198-208 (2001)
A novel validated stability-indicating RP-HPLC method for the determination of Exemestane (steroidal aromatase inhibitor)
Mukthinuthalapati MA and Bukkapatnam V
Journal of Bioequivalence & Bioavailability, 7(6), 288-288 (2015)
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej