Kluczowe dokumenty
PHR1874
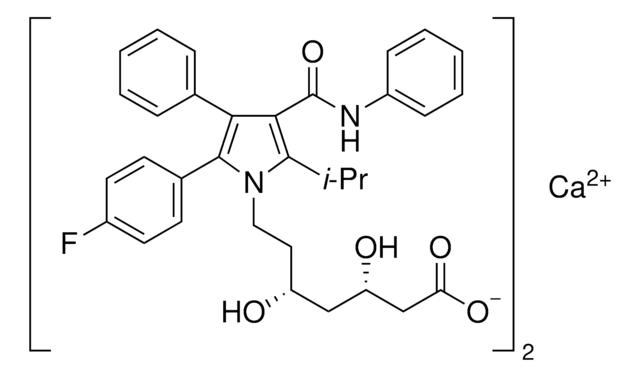
Atorvastatin Related Compound I
pharmaceutical secondary standard, certified reference material
Synonim(y):
tert-Butyl (4R,6R)-6-[2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1-pyrrolyl]ethyl]-2,2-dimethyl-1,3-dioxane-4-acetate, tert-Butyl 2-((4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxan-4-yl)acetate, (4R,6R)-6-[2-[2-(4-Fluorophenyl)-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid 1,1-dimethylethyl ester, tert-Butyl 2-[(4R,6R)-6-[2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetate
About This Item
Polecane produkty
klasa czystości
certified reference material
pharmaceutical secondary standard
Poziom jakości
agency
traceable to USP 1044593
rodzina API
atorvastatin
Certyfikat analizy
current certificate can be downloaded
opakowanie
pkg of 30 mg
mp
144-148 °C (lit.)
Zastosowanie
pharmaceutical
Format
neat
temp. przechowywania
2-8°C
ciąg SMILES
CC(C)c1c(C(=O)Nc2ccccc2)c(-c3ccccc3)c(-c4ccc(F)cc4)n1CC[C@@H]5C[C@H](CC(=O)OC(C)(C)C)OC(C)(C)O5
InChI
1S/C40H47FN2O5/c1-26(2)36-35(38(45)42-30-16-12-9-13-17-30)34(27-14-10-8-11-15-27)37(28-18-20-29(41)21-19-28)43(36)23-22-31-24-32(47-40(6,7)46-31)25-33(44)48-39(3,4)5/h8-21,26,31-32H,22-25H2,1-7H3,(H,42,45)/t31-,32-/m1/s1
Klucz InChI
NPPZOMYSGNZDKY-ROJLCIKYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Zastosowanie
Komentarz do analizy
Inne uwagi
Przypis
produkt powiązany
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumenty section.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej