Kluczowe dokumenty
I7000000
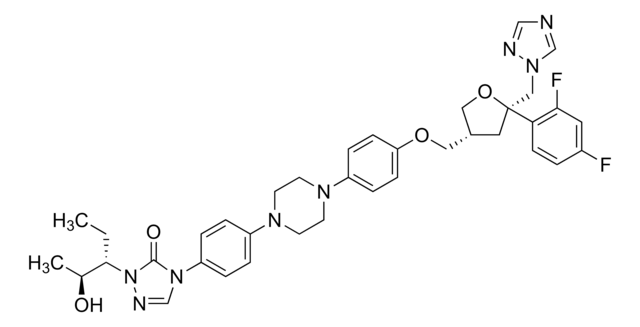
Itraconazole
European Pharmacopoeia (EP) Reference Standard
Synonim(y):
Oriconazole, R51211, Sporanox
About This Item
Polecane produkty
klasa czystości
pharmaceutical primary standard
rodzina API
itraconazole
producent / nazwa handlowa
EDQM
Zastosowanie
pharmaceutical (small molecule)
Format
neat
temp. przechowywania
2-8°C
ciąg SMILES
CCC(C)N1N=CN(C1=O)c2ccc(cc2)N3CCN(CC3)c4ccc(OCC5COC(Cn6cncn6)(O5)c7ccc(Cl)cc7Cl)cc4
InChI
1S/C35H38Cl2N8O4/c1-3-25(2)45-34(46)44(24-40-45)29-7-5-27(6-8-29)41-14-16-42(17-15-41)28-9-11-30(12-10-28)47-19-31-20-48-35(49-31,21-43-23-38-22-39-43)32-13-4-26(36)18-33(32)37/h4-13,18,22-25,31H,3,14-17,19-21H2,1-2H3
Klucz InChI
VHVPQPYKVGDNFY-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
Działania biochem./fizjol.
Opakowanie
Inne uwagi
produkt powiązany
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organy docelowe
Respiratory system
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumenty section.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej