Kluczowe dokumenty
G0326000
Gliclazide
European Pharmacopoeia (EP) Reference Standard
Synonim(y):
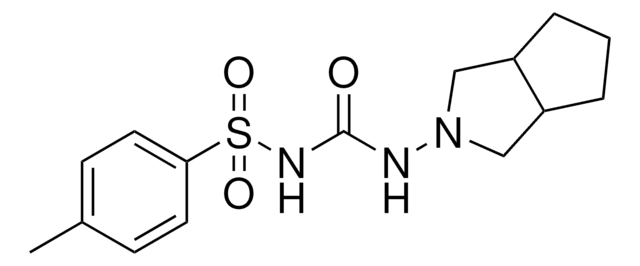
1-(3-Azabicyclo[3.3.0]oct-3-yl)-3-p-tolylsulphonylurea
About This Item
Polecane produkty
klasa czystości
pharmaceutical primary standard
rodzina API
gliclazide
producent / nazwa handlowa
EDQM
mp
163-169 °C (lit.)
Zastosowanie
pharmaceutical (small molecule)
Format
neat
temp. przechowywania
2-8°C
ciąg SMILES
Cc1ccc(cc1)S(=O)(=O)NC(=O)NN2CC3CCCC3C2
InChI
1S/C15H21N3O3S/c1-11-5-7-14(8-6-11)22(20,21)17-15(19)16-18-9-12-3-2-4-13(12)10-18/h5-8,12-13H,2-4,9-10H2,1H3,(H2,16,17,19)
Klucz InChI
BOVGTQGAOIONJV-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
Działania biochem./fizjol.
Opakowanie
Inne uwagi
produkt powiązany
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Oral
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumenty section.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej