TZHVCA210
Steritest® Urządzenie NEO
For liquids in cartridges and small soft plastic containers., Red base canister comes with a single short (20 mm) needle., pkg of 10 blisters per box (Single packed)
Synonim(y):
Urządzenie Red Base Steritest® NEO do testowania sterylności, Urządzenie do testowania sterylności, urządzenie do filtracji membranowej, kanister do filtracji membranowej, zamknięta filtracja membranowa
About This Item
Polecane produkty
Materiały
Nylon 66 adapter (for needle)
PVC tubing (double lumen)
PVDF membrane
stainless steel (for needle)
styrene-acrylonitrile (SAN) (for canister)
agency
EP 2.6.1
JP 4.06
USP 71
sterylność
sterile; γ-irradiated
Właściwości
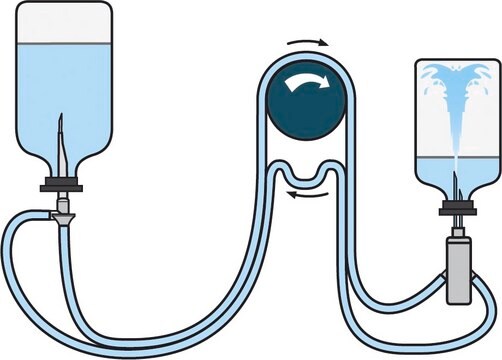
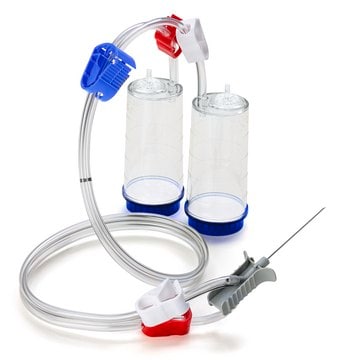
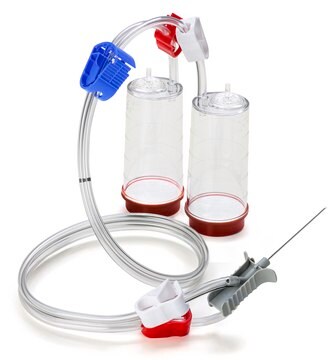
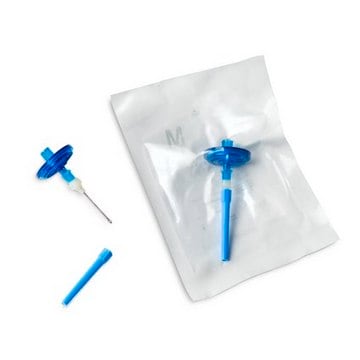
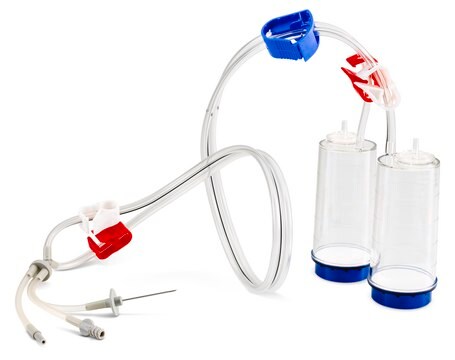
Red base canister comes with a single short (20 mm) needle.
producent / nazwa handlowa
Steritest®
opakowanie
pkg of 10 blisters per box (Single packed)
Parametry
120 mL sample volume (graduation marks at 25, 50, 75 and 100 mL)
3.1 bar max. inlet pressure (45 psi) at 25 °C
45 °C max. temp.
dł. rurki
850 mm
kolor
red Canister Base
Matryca
Durapore®
wielkość porów
0.45 μm pore size
moc wejściowa
liquid
pharmaceutical(s)
Zastosowanie
pharmaceutical
sterility testing
kompatybilność
For liquids in cartridges and small soft plastic containers.
for use with Steritest® Symbio FLEX Pump Kit, 2 media (SYMBFLE01)
for use with Steritest® Symbio ISL Pump Kit, 2 media (SYMBISL01)
for use with Steritest® Symbio LFH Pump Kit (SYMBLFH01)
Opis ogólny
Steritest® NEO to urządzenie do filtracji membranowej do testowania sterylności produktów farmaceutycznych, które można filtrować. Urządzenie upraszcza każdy aspekt testowania, od obsługi po identyfikowalność. Zamknięty system minimalizuje liczbę wyników fałszywie dodatnich i oferuje najwyższy poziom jakości i niezawodności. Urządzenie to zapewnia, że produkty farmaceutyczne nigdy nie są narażone na działanie środowiska podczas procesu testowania. Ten system testowy oferuje zoptymalizowany i w pełni zgodny z przepisami proces testowania, gdy jest używany z pompą Steritest® Symbio, określonymi akcesoriami oraz wysokiej jakości pożywkami i płynami do płukania. Urządzenie Steritest® NEO do płynów w kartridżach zawiera krótką (20 mm) pojedynczą igłę dla łatwego i bezpiecznego dostępu do kartridża lub małego miękkiego plastikowego pojemnika oraz oddzielną igłę odpowietrzającą. Czerwona podstawa pojemnika wskazuje na niską adsorpcję membrany z polifluorku winylidenu (PVDF) 706 i specjalną konstrukcję drenu. Optymalizuje to płukanie produktów, które hamują rozwój drobnoustrojów.
Zastosowanie
Cechy i korzyści
- Kompleksowa obsługa testów sterylności za pomocą naszych urządzeń, pomp, mediów, płynów i usług
- Urządzenia Steritest® są produkowane w naszym Centrum Doskonałości w Molsheim we Francji, z zachowaniem wysokiej jakości standardów kontroli i Certyfikatu Jakości dla każdej partii.
- Nowa konstrukcja igły
- Inteligentniejszy przepływ pracy
- Całkowicie zamknięta konfiguracja
- Stała wydajność
- Nowe narzędzie do odłączania rurek
Opakowanie
Informacje prawne
skonfigurowane dla
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej