PF033
Fas Ligand, Human, Recombinant
Fas Ligand, Human, Recombinant, consists of amino acids 103-281 fused at the N-terminus to a tag-linker peptide. Binds to human, rat, and mouse Fas.
Synonim(y):
CD95L, CD178
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Kod UNSPSC:
12352202
NACRES:
NA.77
Polecane produkty
rekombinowane
expressed in HEK 293 cells
Poziom jakości
Próba
≥95% (SDS-PAGE)
Postać
lyophilized
siła działania
50 ng/mL ED50 (A20 cells)
nie zawiera
preservative
reaktywność gatunkowa
human, mouse, rat
producent / nazwa handlowa
Calbiochem®
warunki przechowywania
OK to freeze
zanieczyszczenia
<0.1 EU/μg Endotoxin (EU/μg purified protein)
Warunki transportu
wet ice
temp. przechowywania
−20°C
Opis ogólny
Recombinant, human, Fas ligand consisting of amino acids 103-281 fused at the N-terminus to a tag-linker peptide. Binds to human, mouse, and rat Fas. The recombinant protein is produced in HEK293 cells. Useful for cytotoxicity assays.
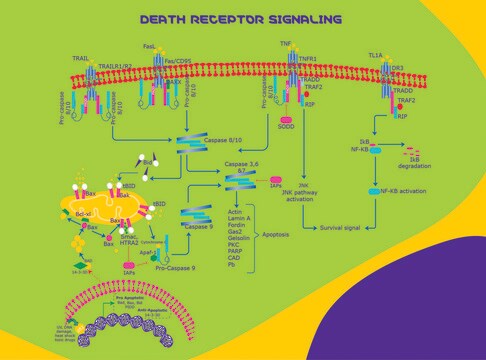
Fas/APO1/CD95 (37-42 kDa), a member of the Tumor Necrosis Factor/Nerve Growth Factor (TNF/NGF) receptor family, is an apoptosis-signaling surface receptor known to trigger apoptotic cell death. Homology exists between the Fas and TNF receptors including a region called the "death domain" which is required to propagate the apoptotic signal. The signal activates a family of cysteine proteases, or caspases, which systematically lead to cell destruction. Genetic defects in the Fas system have been shown to lead to autoimmune disorders while increased activity or deregulation contributes to the pathology of diseases such as AIDS.
Fas/APO1/CD95 (37-42 kDa), a member of the Tumor Necrosis Factor/Nerve Growth Factor (TNF/NGF) receptor family, is an apoptosis-signaling surface receptor known to trigger apoptotic cell death. Homology exists between the Fas and TNF receptors including a region called the "death domain" which is required to propagate the apoptotic signal. The signal activates a family of cysteine proteases, or caspases, which systematically lead to cell destruction. Genetic defects in the Fas system have been shown to lead to autoimmune disorders while increased activity or deregulation contributes to the pathology of diseases such as AIDS.
Recombinant, human, Fas ligand consisting of amino acids 103-281 fused at the N-terminus to a tag-linker peptide. Binds to human, rat, and mouse Fas.The recombinant human soluble Fas ligand recognizes the Fas receptor in human, rat and mouse. Extracellular domain of human FasL, 103 - 281 fused at the N-terminus to a tag linker peptide.
Opakowanie
Please refer to vial label for lot-specific concentration.
Ostrzeżenie
Toxicity: Standard Handling (A)
Postać fizyczna
Lyophilized from PBS.
Rekonstytucja
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
Reconstitute in 50 μl H₂O to yield a final stock concentration of 100 µg/ml. Further dilute in culture medium containing 5% FCS immediately prior to use.
Inne uwagi
Noguchi, K. et al. 1996. Oncogene13, 39.
Golstein, P. et al. 1995. Cell81, 185.
Katsikis, P. et al. 1995. J. Exp. Med.181, 2029.
Nagata, S. et al. 1995. Science267, 1449.
Thompson, C., 1995. Science267, 1456.
Watanabe-Fukunaga, R. et al. 1992. Nature356, 314.
Golstein, P. et al. 1995. Cell81, 185.
Katsikis, P. et al. 1995. J. Exp. Med.181, 2029.
Nagata, S. et al. 1995. Science267, 1449.
Thompson, C., 1995. Science267, 1456.
Watanabe-Fukunaga, R. et al. 1992. Nature356, 314.
The soluble Fas ligand kills APO-1/Fas-sensitive cells at a concentration of >50 ng/ml. For example, cell death results when 5 x 105 murine A20 B lymphoma cells are incubated with 50-500 ng/ml of the recombinant human soluble APO1/FasL (aa 103-281) for 16 h at 37°C.
Informacje prawne
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
This page may contain text that has been machine translated.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 1
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej