MABN878
Anti-phospho-Amyloid beta (Ser8) Antibody, clone 1E4E11
clone 1E4E11, from mouse
Synonim(y):
Aβ peptide, Ser8 phosphorylated, Abeta peptide, Ser8 phosphorylated, Amyloid β peptide, Ser8 phosphorylated, Aβ1-40, Ser8 phosphorylated, Aβ1-42, Ser8 phosphorylated, Abeta1-40, Ser8 phosphorylated, Abeta1-42, Ser8 phosphorylated
About This Item
Polecane produkty
pochodzenie biologiczne
mouse
Poziom jakości
forma przeciwciała
purified immunoglobulin
rodzaj przeciwciała
primary antibodies
klon
1E4E11, monoclonal
reaktywność gatunkowa
human
metody
ELISA: suitable
immunofluorescence: suitable
immunohistochemistry: suitable
western blot: suitable
izotyp
IgG1κ
numer dostępu NCBI
numer dostępu UniProt
docelowa modyfikacja potranslacyjna
phosphorylation (pSer8)
informacje o genach
human ... APP(351)
Opis ogólny
Specyficzność
Immunogen
Zastosowanie
Neuroscience
Neurodegenerative Diseases
Western Blotting Analysis: 2 µg/mL from a representative lot detected phosphorylated oligomeric amyloid beta (pAβ) peptides in 50 µg of brain extract from an 8-month old APP/PS1KI transgenic mouse, but not in extract from an age-matched non-transgenic mouse (Courtesy of Dr. Kumar and Prof. Dr. Walter, Department of Neurology, University Bonn, Germany).
ELISA Analysis: Clone 1E4E11 hybridoma culture supernatant detected the immunogen peptide with phosphorylated Ser8, but not the corresponding non-phosphorylated peptide (Kumar, S., et al. (2013). Acta Neuropathol. 125(5):699-709).
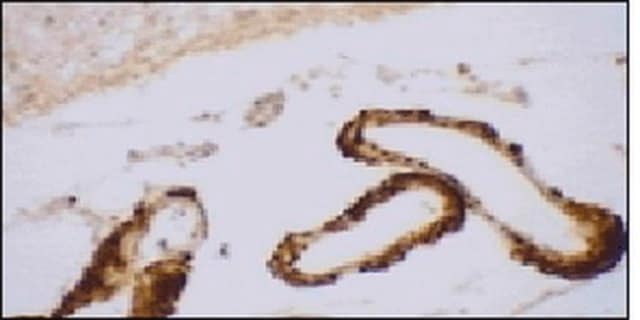
Immunohistochemistry Analysis: A representative lot detected age-dependent phospho-amyloid beta (pAβ) immunoreactivity and localization in paraffin-embedded APP/PS1KI mouse brain sections following antigen retrieval by heat and 88% formic acid treatments (Kumar, S., et al. (2013). Acta Neuropathol. 125(5):699-709).
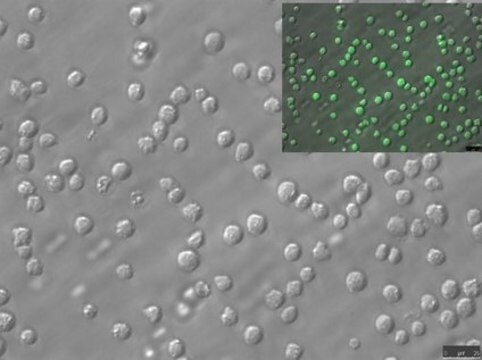
Immunofluorescence Analysis: A representative lot detected phospho-amyloid beta (pAβ) immunoreactivity in cortical neurons colocalized with that detected with a non-phospho-specific Aβ antibody by dual fluorescent immunohistochemistry staining of paraffin-embedded 2-month old APP/PS1KI mice cotex sections following anigen retrieval by heat and 88% formic acid treatments (Kumar, S., et al. (2013). Acta Neuropathol. 125(5):699-709).
Western Blotting Analysis: A representative lot detected monomeric, dimeric, trimeric, and oligomeric forms of synthetic Aβ1-40 and Aβ1-42 peptides with phosphorylated Ser8, but not the non-phosphorylated Aβ1-40 and Aβ1-42 peptides (Kumar, S., et al. (2013). Acta Neuropathol. 125(5):699-709).
Western Blotting Analysis: A representative lot detected monomeric, dimeric, and oligomeric forms of phosphorylated amyloid beta (pAβ) peptides in brain extracts from 6- and 12-month old APP/PS1KI transgenic mice, but not in brain extracts from age-matched non-transgenic mice (Kumar, S., et al. (2013). Acta Neuropathol. 125(5):699-709).
Note: Formic acid (88%) treatment following heat retrieval is recommended for immunohistochemical detection of aggregated intraneuronal Abeta peptides in brain sections (Kumar, S., et al. (2013). Acta Neuropathol. 125(5):699-709; Christensen, D.Z., et al. (2009). Brain Res. 1301:116-125).
Jakość
Isotyping Analysis: The identity of this monoclonal antibody is confirmed by isotyping test to be IgG1κ.
Opis wartości docelowych
Postać fizyczna
Przechowywanie i stabilność
Inne uwagi
Oświadczenie o zrzeczeniu się odpowiedzialności
Nie możesz znaleźć właściwego produktu?
Wypróbuj nasz Narzędzie selektora produktów.
Kod klasy składowania
12 - Non Combustible Liquids
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej