Opis ogólny
A cell-permeable compound that specifically, reversibly, and stoichiometrically (1:1) binds to the GDP-bound Ral A/B in an allosteric manner (Kd = 7.7 µM) and blocks its activation.
A cell-permeable dihydropyranopyrazole compound that binds and locks RalA/B in the inactive GDP-bound form by targeting an allosteric site close to the guanine nucleotide-binding pocket (Kd = 7.7 µM binding study by ITC using RalB) in a 1:1 stoichiometric ratio, while exhibiting little affinity toward free or GTP-bound Ral. Shown to inhibit anchorage-independent growth of human lung cancer cell lines H358 & H2122 in vitro (IC50 = 1.3 & 2.0 µM, respectively, in 2-4 wks by soft agar colony formation assays) by reducing cellular level of ATP-bound, active RalA/B (by >90% with 10 µM BQU57 treatment for 3 h; assessed by by RALBP1-agarose pull-down). BQU57 is bioavailable in mice via intraperitoneal injection (Plasma T1/2 = 1.5 h, AUC0-5 h = 28.6 µg·h/mL post single 50 mg/kg i.p. dosage) with overall tissue distribution, including brain (µg drug/g tissue = 4.1/liver, 2.4/kidney, 2.1/heart, 1.8/lung, 1.4/brain, 1.2/H2122 tumor; 3 h post single 50 mg/kg i.p. dosage). Shown to selectively downregulate leves of active RalA/B, but not Ras or RhoA, in H2122-derived tumor in mice in a dose-dependent manner (64% and 86% reduction of ATP-bound RalA and RalB, respectively, 3 h post single 50 mg/kg i.p. dosage) and effectively suppress H2122 tumor expansion when administered via daily i.p. dosing (by 70% 22 d post H2122 inoculation with 21 daily 50 mg/kg/d i.p. dosages) in vivo.
A cell-permeable dihydropyranopyrazole compound that binds and locks RalA/B in the inactive GDP-bound form by targeting an allosteric site close to the guanine nucleotide-binding pocket (Kd = 7.7 µM binding study by ITC using RalB) in a 1:1 stoichiometric ratio, while exhibiting little affinity toward free or GTP-bound Ral. Shown to inhibit anchorage-independent growth of human lung cancer cell lines H358 & H2122 in vitro (IC50 = 1.3 & 2.0 µM, respectively, in 2-4 wks by soft agar colony formation assays) by reducing cellular level of ATP-bound, active RalA/B (by >90% with 10 µM BQU57 treatment for 3 h; assessed by by RALBP1-agarose pull-down). BQU57 is bioavailable in mice via intraperitoneal injection (Plasma T1/2 = 1.5 h, AUC0-5 h = 28.6 µg·h/mL post single 50 mg/kg i.p. dosage) with overall tissue distribution, including brain (µg drug/g tissue = 4.1/liver, 2.4/kidney, 2.1/heart, 1.8/lung, 1.4/brain, 1.2/H2122 tumor; 3 h post single 50 mg/kg i.p. dosage). Shown to selectively downregulate leves of active RalA/B, but not Ras or RhoA, in H2122-derived tumor in mice in a dose-dependent manner (64% and 86% reduction of ATP-bound RalA and RalB, respectively, 3 h post single 50 mg/kg i.p. dosage) and effectively suppress H2122 tumor expansion when administered via daily i.p. dosing (by 70% 22 d post H2122 inoculation with 21 daily 50 mg/kg/d i.p. dosages) in vivo.
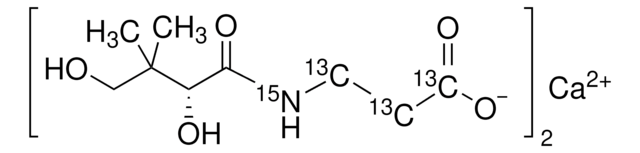
Please note that the molecular weight for this compound is batch-specific due to variable water content.
Rekonstytucja
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
Use only fresh DMSO for reconstitution.
Inne uwagi
Yan, C., et al. 2014. Nature.515, 443.