Key Documents
Z-005
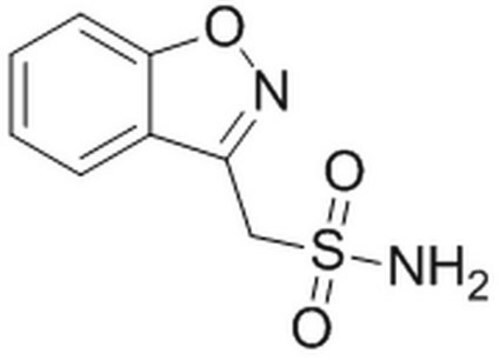
Zonisamide solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Polecane produkty
klasa czystości
certified reference material
Postać
liquid
Właściwości
Snap-N-Spike®/Snap-N-Shoot®
opakowanie
ampule of 1 mL
producent / nazwa handlowa
Cerilliant®
stężenie
1.0 mg/mL in methanol
metody
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
Zastosowanie
clinical testing
format
single component solution
temp. przechowywania
−20°C
ciąg SMILES
NS(CC1=NOC2=C1C=CC=C2)(=O)=O
InChI
1S/C8H8N2O3S/c9-14(11,12)5-7-6-3-1-2-4-8(6)13-10-7/h1-4H,5H2,(H2,9,11,12)
Klucz InChI
UBQNRHZMVUUOMG-UHFFFAOYSA-N
informacje o genach
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
Opis ogólny
Zastosowanie
- Analysis of environmental samples: Instrument Response Standard for X-Ray Powder Diffraction was utilized for the dispersive magnetic micro solid phase extraction of pollutants in water samples and creams. This technique is vital for scientists engaged in environmental monitoring and contamination studies (Jalilian et al., 2019).
- Food safety testing: The standard supported efficient extraction and determination methods for Rhodamine B in food samples, underscoring its importance in food safety and quality control labs focused on consumer health protection (Hu et al., 2019).
- Advanced material characterization: A core-shell indium (III) sulfide@metal-organic framework nanocomposite used for the extraction of nitro-polycyclic aromatic hydrocarbons employs Instrument Response Standards to ensure accurate characterization of nanocomposite properties, critical for researchers developing new materials (Jia et al., 2018).
Informacje prawne
produkt powiązany
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Organy docelowe
Eyes
Kod klasy składowania
3 - Flammable liquids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
49.5 °F - closed cup
Temperatura zapłonu (°C)
9.7 °C - closed cup
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej