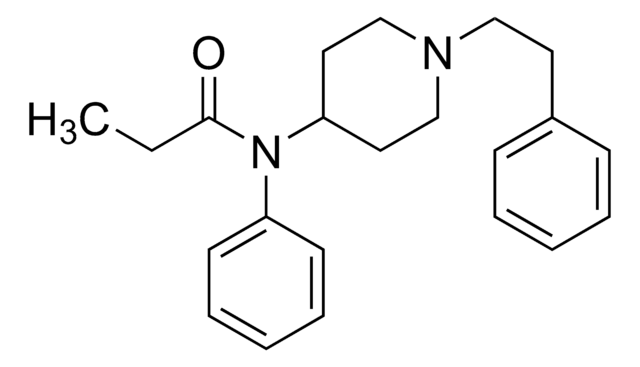
P-011
(+)-Propoxyphene solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Polecane produkty
klasa czystości
certified reference material
Postać
liquid
Właściwości
Snap-N-Spike®/Snap-N-Shoot®
opakowanie
ampule of 1 mL
producent / nazwa handlowa
Cerilliant®
drug control
Narcotic Licence Schedule A (Switzerland); estupefaciente (Spain); Decreto Lei 15/93: Tabela IA (Portugal)
stężenie
1.0 mg/mL in acetonitrile
metody
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
Zastosowanie
forensics and toxicology
format
single component solution
temp. przechowywania
2-8°C
ciąg SMILES
CCC(=O)O[C@@](Cc1ccccc1)([C@H](C)CN(C)C)c2ccccc2
InChI
1S/C22H29NO2/c1-5-21(24)25-22(18(2)17-23(3)4,20-14-10-7-11-15-20)16-19-12-8-6-9-13-19/h6-15,18H,5,16-17H2,1-4H3/t18-,22+/m1/s1
Klucz InChI
XLMALTXPSGQGBX-GCJKJVERSA-N
informacje o genach
human ... OPRM1(4988)
Opis ogólny
Informacje prawne
produkt powiązany
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Kod klasy składowania
3 - Flammable liquids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
35.6 °F - closed cup
Temperatura zapłonu (°C)
2 °C - closed cup
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej