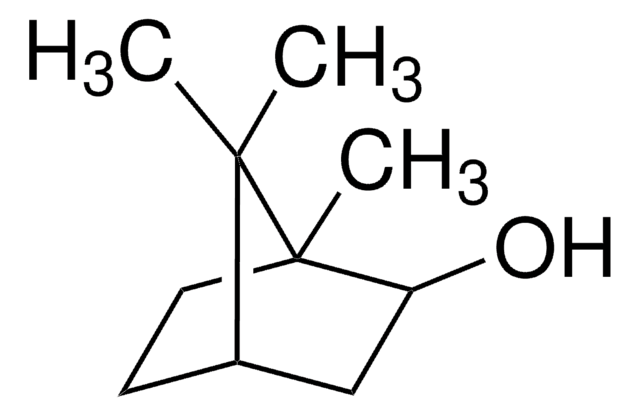
Key Documents
W248010
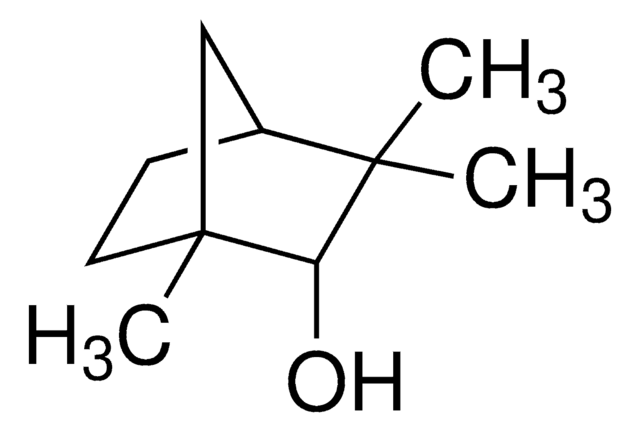
Fenchyl alcohol
≥96%, FG
Synonim(y):
Fenchol
About This Item
Polecane produkty
pochodzenie biologiczne
synthetic
klasa czystości
FG
Halal
Kosher
zgodność regionalna
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
Próba
≥96%
mp
39.0-45.0 °C (lit.)
Zastosowanie
flavors and fragrances
Dokumentacja
see Safety & Documentation for available documents
alergen pokarmowy
no known allergens
Organoleptyczne
earthy; camphoraceous; pine
ciąg SMILES
[H][C@]12CC[C@](C)(C1)[C@@H](O)C2(C)C
InChI
1S/C10H18O/c1-9(2)7-4-5-10(3,6-7)8(9)11/h7-8,11H,4-6H2,1-3H3/t7-,8-,10+/m0/s1
Klucz InChI
IAIHUHQCLTYTSF-OYNCUSHFSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
- Enhancing the NMR signals of plant oil components using hyperpolarisation relayed via proton exchange.: This study explores the enhancement of NMR signals of plant oil components, including fenchyl alcohol, using hyperpolarization techniques, which significantly improve the detection sensitivity in NMR spectroscopy (Alshehri et al., 2023).
- Update to RIFM fragrance ingredient safety assessment, fenchyl alcohol, CAS Registry Number 1632-73-1.: This paper provides an updated safety assessment of fenchyl alcohol as a fragrance ingredient, focusing on its toxicological profile and safe usage levels (Api et al., 2022).
- Olfactory Impact of Terpene Alcohol on Terpenes Aroma Expression in Chrysanthemum Essential Oils.: The research investigates how fenchyl alcohol and other terpene alcohols influence the aroma profile of chrysanthemum essential oils, highlighting its significant role in enhancing aromatic properties (Niu et al., 2018).
- Antibiofilm and Antihyphal Activities of Cedar Leaf Essential Oil, Camphor, and Fenchone Derivatives against Candida albicans.: This study demonstrates the antibiofilm and antihyphal activities of fenchyl alcohol derivatives against Candida albicans, suggesting potential applications in antifungal treatments (Manoharan et al., 2017).
- Comparative Study on Volatile Compounds of Alpinia japonica and Elettaria cardamomum.: The paper compares the volatile compounds of Alpinia japonica and Elettaria cardamomum, identifying fenchyl alcohol as a key constituent, and discusses its implications for flavor and fragrance applications (Asakawa et al., 2017).
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organy docelowe
Respiratory system
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
165.2 °F - closed cup
Temperatura zapłonu (°C)
74 °C - closed cup
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej